Keywords
Bananas; Phenolic compounds; Antioxidant activity; DPPH; FRAP
Introduction
Over the recent decades, phenolic compounds have been a subject of high interest owing to their possible association with intake and the prevention of these major chronic diseases as suggested by clinical trials and epidemiological studies [1]. The increase in intensity and scope of investigations with respect to the phenolic compounds has been due to their high spectrum of biological activities, including antioxidant, anti-inflammatory, antibacterial, anti-cancer and antiviral functions [2].
Despite the human body having effective defense mechanisms to safeguard the cellular molecules against ROS induced damage via mainly enzymatic and non-enzymatic antioxidant systems, these natural defenses are most likely inadequate for severe or continued oxidative stress notwithstanding that these system’s protective ability decreases with age. Consequently, in order to maintain adequate amounts of antioxidants in the human body, there is need to utilize external source of antioxidants via the normal diet or supplements.
Bananas are among the most popular fruits on the world market. Uganda ranks only behind India in banana production in the world with about 12 million tons per annum (https:// www.worldlistmania.com/worlds-largest-bananas-producingcountries/) and most of it is consumed locally thus neglibly contributing to world banana exports. Various studies have evidenced that both banana pulp and peel contain various phenolic compounds such as catechin, epicatechin, lignin and tannins and anthocyanins [3]. A study [4] reported that in general the peel extracts of eight Malaysian bananas (Musa sp.) exhibited higher total phenolic content and stronger antioxidant activities than their corresponding pulps. Due to broadening spectrum of polyphenol applications and the desire by various governments in developing countries as well as development agencies to promote regional economies and sustainable agricultural activities, diversification of the use of locally available material has been sought in recently.
Bananas have been reported to contain considerable amounts of bioactive phytochemicals in the fruit pulp with the average values for the total phenolic compounds ranging from 44.46 to 52.02 mg of catechin equivalent/100 g of fresh weight, respectively [5,6]. Whereas phenolic compounds from many fruits have been intensively studied, comparatively few works on the phenolic composition and/or content of banana fruits have been carried out such as: The total amount of phenolic compounds in banana (Musa acuminata Colla AAA) peel was reported to range from 0.90 to 3.0 g/100 g DW [7]. A study [3] identified gallocatechin in the banana (Musa Cavendish), which was found more abundant in the peel (158 mg/100 g dry wt) than in pulp (29.6 mg/100 g dry wt). Banana pulp was also reported to have phenolic compounds like catechin and epicatechin [7]. A study [8] reported that the banana peel contained a greater range of phenolics composition. The rhizomes of commercial banana cultivars grown in India were reported to contain phenolic compounds such as cinnamic acid, gentisic acid, protocatechuic acid, caffeic acid, ferulic acid, (+) catechin and chlorogenic acid, catechol, gallic acid, chlorogenic acid, tannic acid, catechol and gallic acid in the different extracts used for extraction [9]. A study [10] reported that, (+) catechin, gallocatechin, and (-) epicatechin, as well as condensed tannins, were detected in the soluble extract of the banana fruit pulp, two hydroxycinnamic acid derivatives were detected in the soluble cell wall fraction, whereas in the insoluble cell wall fraction of bananas, the anthocyanidin delphinidin was reported to be predominant.
Although phenolic compounds from some banana varieties have been identified, it is well documented that difference in nutrient composition within even a given banana variety vary considerably with respect to: region of production, farming method (Organic or inorganic), etc. (Forster et al. (2002). Since to our knowledge, a comparative evaluation of the polyphenolic compounds and antioxidant activities of banana varieties grown in Uganda such as the Kivuvu (ABB) and Gonja (AAB) has not been reported to date and there is promotion of locally available material as sources of antioxidants, it was found imperative to determine the total polyphenolic content, phenolic compounds and antioxidant capacity of the Kivuvu (ABB) and Gonja (AAB) banana varieties grown in Uganda.
Therefore, this study is of importance as the nutrient composition of fruits and vegetables varies greatly with respect to soil type, varieties, environmental conditions, latitude, agronomic practices, etc. Further the polyphenolic profiles of fruits are dependent on maturity, cultivars, geographical origin, growing season, postharvest storage condition and processing techniques.
Characterization of phenolic compounds in different banana varieties is very important, since phenolics play a significant role in the prevention of many chronical diseases. Additionally, though the banana peel is not an edible part of the fruit, the knowledge of phenolic composition is valuable, since it could be used as a matrix to extract phenolic compounds which would transform it into a valuable product. The young bananas were included in the study to determine their potential as a source of antioxidant in form of phenolic compounds with the aim of reducing on the losses the banana farmers incur due to heavy wind storms, rain and hailstorms which are becoming rampant with climatic change effects.
Hypothesis: The same antioxidant activity levels and phenolic profiles are found in the Kivuvu (ABB) and Gonja (AAB) banana varieties grown in Uganda with respect to plant part and stage of growth.
Materials and Methods
Plant materials
The roasting bananas plantain type (Musa acuminata) AAB genotype) known locally as Gonja and the cooking/juice type bananas (Musa acuminata) ABB genotype known locally as Kivuvu were used throughout this investigation were grown at the PIBID garden in Bushenyi district in Uganda. The mature and young Gonja banana (AAB genotype) and the mature and young Kivuvu bananas (ABB) were used in this study and mature or ready for harvest stage was at 15-18 weeks from flowering while the young stage was 4-6 weeks from flowering which was adopted from [11]. The fresh bananas were harvested and after 48 hrs of transportation, they were kept at -80°C until needed for analyses.
Chemicals and reagents
Gallic acid, Folin-Ciocâlteu reagent, Trolox standard (6-hydroxy- 2,5,7,8,-tetramethyl-chroman-2-carboxylic acid), Sodium ascorbate, 2,4,6–tri (4-pyridyl)-1,3,5-triazine (TPTZ), HCl, Fecl3.6H2O, FeSO4, 1,1-diphenyl-2-picrylhyrazyl (DPPH), Gallic acid, were purchased from Wako Pure Chemical Industries Ltd, Osaka, Japan. All other reagents were of analytical grade. Standard solutions were prepared with deionized water.
Extraction of polyphenols
About 2.0 g of the frozen banana pulp and peel samples were taken and weighed directly in Pyrex tubes, containing 20 ml of acidified ethanol 70% (1% of 1 N hydrochloric acid, pH 3.0) in 1:10 ratio (solid: solvent). The use of acidified solvents has been used by various researchers [12] to extract phenolic compounds from plant materials and was thus adopted. The samples were immediately homogenized and extracted in a shaking incubator for 2 hrs. After completion of the extraction time, the crude extract was centrifuged at 3000 rpm for 15 min. The supernatant was collected and kept at -80°C until further analyses.
Total phenolic content analysis
Total phenolic content of the banana extracts was determined using Folin-Ciocâlteu method described by a study [13] with modifications. 1 mL of banana extract was added to a glass tube using a pipette and 9 mL of deionized water was added to give a dilution of 10 times. 1 mL of the diluted banana extract was taken to another glass tube using a pipette and 1 mL of Folin-Ciocâlteu reagent added and mixed well. 1 mL of 10% sodium carbonate was added within a period of 1 to 7.59 min. Then, 4 mL of deionized water was added to the glass tube containing the solution and incubated at room temperature in the dark for 2 hrs. After the 2 hrs, Absorbance’s of the solutions were measured at 760 nm using a UV- Vis spectrophotometer (V-560, Jasco International Co. Ltd, Tokyo, Japan) and the result expressed in mg of Gallic Acid Equivalents (mg/100 g). A calibration curve was prepared, using a standard solution of gallic acid (20, 40, 60, 80 and 100 mg/l, r2=0.997). All measurements were done in triplicates.
DPPH radical scavenging activity
The hydrogen-donating or radical scavenging ability of the banana extracts was determined by DPPH according to [14] method with modifications. Aliquots of 1.50 mL of sample were added to aliquots of 2.85 mL of 0.1 mM methanolic DPPH solution into clean plastic vials. A blank with 1.50 mL of pure methanol was run to obtain a correction factor (due to dilution). The sample-DPPH solutions were left for 1 h in the dark at room temperature to react and at 5 min intervals a solution was transferred to a clean cuvette to take spectrophotometer reading at 515 nm. Percentage of inhibition of the DPPH radical was calculated according to the following equation:

Where Abs control is the absorbance of DPPH solution without extracts.
Also for the determination of antioxidant activity using DPPH in the extracts, absorbance measurements commenced immediately after mixing the extract with the DPPH solution and the decrease in absorbance at 515 nm was determined continuously at 60 seconds intervals with UV-Vis spectrophotometer until the reaction reached a plateau.
Ferric-reducing antioxidant power assay (FRAP assay)
FRAP assay was performed according to a modified method described [15-32]. In brief, 0.15 ml of banana extracts were allowed to react with 2.85 ml of FRAP solution for 30 min in the dark. Then, the reaction mixture was incubated at 37°C for 4 min prior to the absorbance reading. Afterwards, spectrophotometric readings of the ferrous tripyridyltriazine complex were taken at 593 nm against a blank that was prepared using distilled water and incubated for 1 h instead of 4 min. FRAP reagent was always pre-warmed at 37°C and freshly prepared by mixing 2.5 ml of a 10 Mm 2, 4, 6-tris (1-pyridyl)-5-triazine (TPTZ) solution in 40 mM HCl with 2.5 ml of 20 mM FeCl3.6H2O and 25 ml of 0.3 M acetate buffer, pH 3.6. FeSO4 was used to generate the calibration curve and results were expressed in μM (Fe (II)/g dry mass with sodium ascorbate as a standard.
HPLC-ESI/MS elucidation of phenolic compounds structures
The protocol used by RIKEN centre for Sustainable Resource Science: Metabolomics Research group, LC-MS branch, Japan was used for the phenolic compound identification [15]. The sample extracts (2 μl) were analyzed using an LC-MS system equipped with an electro spray ionization (ESI) interface (HPLC: Waters Acquity UPLC system; MS: Waters Q-Tof Premier). The analytical conditions were as follows. HPLC: column: Acquity BEH C18 (pore size: 1.7 microm), Waters, 2.1 by 100 mm; solvent system: acetonitrile (0.1% formic acid):water (0.1% formic acid); gradient program: 1:99, v/v, at 0 min; 1:99, v/v, at 0.1 min; 99.5:0.5 at 15.5 min; 99.5:0.5 at 17.0 min; 1:99, v/v, at 17.1 min; and 1:99 at 20 min; flow rate: 0.3 ml/min; temperature: 38°C. MS detection: capillary voltage: +3.0 keV; cone voltage: 35 V; source temperature: 120°C; desolvation temperature: 450°C; cone gas flow: 50 l/h; desolvation gas flow: 800 l/h; collision energy: ramp 5-45 V; detection mode: negative polarity [M-H]-, scan (m/z 100- 2000; dwell time: 0.45 s; inters can delay: 0.05 s, centroid). The scans were repeated for 19.5 min in a single run. The data were recorded with the aid of MassLynx version 4.1 software (Waters). The phenolic compounds present in the banana extracts were characterized according to their UV, retention times and ESIMS spectra compared with those of known plant metabolites in the RRIMe and Mass bank databases as well as from literature.
Statistical analysis
Data were analyzed using GenStat software 12th Edition. Analysis of variance (ANOVA) was used to compare any significant differences between solvents and samples. Values were expressed as means ± standard deviations. Differences were considered significant at P < 0.05. All the analyses were carried out in at least triplicates.
Results and Discussion
Total polyphenol content of Gonja and Kivuvu bananas
Table 1 shows the total phenolic content of the banana fruit extracts of Gonja (AAB) and Kivuvu varieties (ABB) measured using Folin-Ciocalteu’s method. The Total Polyphenol content of the banana fruits ranged from 42.85 ± 0.80 GAE mg/100 g (dwb) to 414.94 ± 31.95 GAE mg/100 g (dwb) for the banana peel and pulp of Gonja (AAB) variety and 54.04 ± 0.24 GAE mg/100 g (dwb) to 523.60 ± 9.05 GAE mg/100 g (dwb) for the banana peel and pulp of Kivuvu (ABB) variety.
Table 1 Total phenolic content and Antioxidant Activity of 70% ethanolic extracts of mature and young Gonja (AAB) and Kivuvu (ABB) banana varieties.
| Banana Sample |
Total polyphenol content (GAE mg/100 g) DWB |
(%) DPPH- inhibition |
FRAP (µmol Fe (II)/g fresh weight) |
| Mature Gonja Pulp |
42.85 ± 0.80a |
71.2 ± 1.71a |
2.70 ± 0.24a |
| Mature Gonja Peel |
182.74 ± 1.86b |
81.1 ± 2.50b |
3.62 ± 0.59b |
| Mature Kivuvu Pulp |
54.04 ± 0.24a |
69.8 ± 1.47a |
1.91 ± 0.29c |
| Mature Kivuvu Peel |
159.77 ± 8.69c |
71.3 ± 1.78a |
2.67 ± 0.31a |
| Young Gonja Pulp |
414.94 ± 31.95d |
80.3 ± 4.12b |
3.20 ± 0.66b |
| YoungGonja Peel |
150.48 ± 16.17c |
69.2 ± 3.46a |
2.59 ± 0.47a |
| Young Kivuvu Pulp |
523.60 ± 9.05e |
84.8 ± 4.78b |
3.80 ± 0.81b |
| Young Kivuvu Peel |
199.61 ± 14.68b |
72.8 ± 4.13a |
2.83 ± 0.45a |
Values are means (n=3) ± SD. Values with the same superscript letter are not statistically significant at the 5% level.
The results for the analysis of variance for the total polyphenol content revealed that there is interaction between banana maturity and banana variety, banana maturity and banana fruit part, and banana variety and banana fruit parts. The results for the interaction between banana maturity and banana variety with respect to total polyphenol content revealed that the bananas with lower maturity levels (young) had higher total phenolic content than the bananas with higher maturity levels (mature) for both varieties that were under study. Furthermore, for both banana varieties, Gonja (AAB) banana variety had higher total polyphenol content than the Kivuvu (ABB) banana variety when the maturity levels were high (mature) while for the young or low maturity levels Kivuvu (ABB) banana variety had higher total polyphenol content than Gonja (AAB).
The results for the interaction between banana maturity and banana fruit part with respect to total polyphenol content revealed that the banana peels’ total polyphenol content did not differ so much in the bananas with lower maturity levels (young) and the bananas with higher maturity levels (mature). However, the banana pulps’ total polyphenol content differed so much in the bananas with lower maturity levels (young) and the bananas with higher maturity levels (mature), with the former having very high amounts of total polyphenol content.
For the mature bananas, the peel had higher total polyphenolic content which was about 3 fold the amount of total polyphenolic content in comparison to that determined in the pulp for both banana varieties. However, for the young bananas, a reverse trend was observed as the pulp had about 10 fold the amount total polyphenol content in comparison to the peel for each banana variety. The peel of mature Gonja (AAB) had higher total phenolic content (182.74 ± 1.86 GAE mg/100 g (dwb)) than the peel of mature Kivuvu (ABB) (159.77 ± 8.69 GAE mg/100 g (dwb)), while the pulp for mature Gonja (AAB) had relatively lower total phenolic content (42.85 ± 0.80 GAE mg/100 g (dwb)) than the mature Kivuvu (ABB) pulp (54.04 ± 0.24 GAE mg/100 g (dwb)).
However, the phenolic content amounts obtained in this study were higher (42.85 ± 0.80-523.60 ± 9.05 GAE mg/100 g (dwb) for the banana peel and pulp of Gonja(AAB) and Kivuvu (ABB) varieties) than those reported by other researchers for other banana types [3] reported total phenolic content of banana (Musa Cavendish) peel to be 0.91 g/100 g DW, while [6] found total phenolic contents of 0.9 and 3.0 g/100 g DW in the ‘‘KluaiKhai” and ‘‘KluaiHom Thong” cultivars, respectively. A study [16] recorded that the TPC of two banana varieties, namely Cavendish and Dream widely ranged from 75.01 to 685.57 mg GAE/100 g of dry matter with TPC values of banana peels being higher than those of banana pulp.
Antioxidant capacity of banana polyphenols
Due to the complexity of the mechanisms through which the photochemical derive their ant oxidative abilities, it is only realistic that no single antioxidant analytical method has the ability to provide a comprehensive depiction of the antioxidant profile of the sample under study [9]. Therefore more than one method was used to study the antioxidant activity of banana Gonja (AAB) and banana Kivuvu (ABB) extracts.
The antioxidant capacity of extracts of Gonja (AAB) and Kivuvu (ABB) banana varieties at two maturity levels was initially measured through the DPPH radical scavenging assay (Table 1). The results depicted the potential of the banana Gonja (AAB) and Kivuvu (ABB extracts as good antioxidants with mean values of 69.2 to 81.1 and 69.8 to 84.8% inhibition for the peels and pulp respectively for DPPH. The results showed that generally the bananas under study had a high antioxidant activity irrespective of their maturity and plant part. For the mature banana samples, the peels exhibited higher mean DPPH values (71.3-81.1% Inhibition) than the respective pulps (69.8-71.2 % Inhibition) an indication of higher free radical scavenging ability. However, in the young banana samples, the pulp had higher mean DPPH values (80.3-84.8% Inhibition) than the respective peels indicating higher free radical scavenging ability of the young bananas’ pulps. A general comparison between the two banana varieties that were investigated revealed that mature, Gonja (AAB) possessed a higher radical scavenging potential than the Kivuvu (ABB) banana variety for both the peel and pulp while the young Kivuvu (ABB) exhibited higher radical scavenging potential than Gonja (AAB) banana for both peel and pulp. [9] Reported that the DPPH activities of the banana rhizomes extracts to be very high i.e. 92.48% at 300 mg/ml which was very close to the activity of standard BHT (93.21%). The maximum activity of DPPH was 19.39 ± 0.15 mg TE/g d.w for the chloroform extract of dried peels of Mas cultivar [4]. Meanwhile, the highest activity of FRAP was shown by most of the chloroform extracts of dried pulps, dominated by Awak cultivar (22.57 ± 0.13 mg TE/g d.w.). The inhibition of DPPH of the extracts from Cavendish and dream varieties ranged from 26.55 to 52.66% [16] The same researchers further reported that Dream banana peel extracts exhibited antioxidant activities ranging from moderate to high despite having appeared to have low TPC and TFC. Banana peel extracts from ‘Gruesa” and ‘‘Grande Naine” were found to demonstrate strong scavenging activity against DPPH [17].
The reducing capacities of the extracts were evaluated through FRAP assay (Table 1). The banana Gonja (AAB) and Kivuvu (ABB extracts exhibited mean values of 1.91 to 3.80 and 2.67 to 3.62 μmol Fe (II)/g fresh weight for the pulp and peels respectively for FRAP assay. The FRAP values also further confirmed the high antioxidant activity of the bananas that were studied. The results were in agreement with earlier reports on banana antioxidant activity. [18] Reported that the antioxidant activity of the methanolic extracts of banana peel was considerably high (83 ± 0.70%). Both mature banana varieties’ peels exhibited higher antioxidant activity as measured by FRAP than the pulp and a reverse trend was shown for the young banana samples. In the young banana samples, Kivuvu (ABB) exhibited higher antioxidant activities as measured by FRAP for both pulp and peel than the corresponding parts for the Gonja (AAB) variety while in the mature banana samples a reverse trend was observed.
A study [4] revealed that high activity with respect to FRAP was exhibited by the chloroform extracts of dried pulps of Awak and Berangan, and dried peels of Rastali with values of 22.57 ± 0.13, 22.53 ± 0.12 and 21.63 ± 0.42 mg TE/g d.w., respectively.
From the antioxidant activity results, the DPPH and FRAP results exhibited a similar pattern for the banana samples and this may be attributed to the two methods having similar mechanisms of free radical scavenging. The high antioxidant activity of the mature Gonja (AAB) peels and young Kivuvu (ABB) pulp were further supported by the presence of Quercetin glycosides and Kaempferol glycosides (Table 2) in the mature Gonja (AAB) peels and Quercetin-3,4’-O-di-beta-glucopyranoside, Kaempferol glycosides and Eriodictyol (Table 2) in the young Kivuvu (ABB) pulp
Table 2 Retention time (RT) at 280 nm wavelength, pseudo molecular ions, MS2fragnment ions (with their relative abundance in brackets) and identification of the phenolic compounds of bananas of Gonja and Kivuvu varieties.
| Banana sample |
HPLC tR (min) |
Molecular ion [M-H]- (m/z) |
MS2 (m/z) (Relative Intensity) |
Tentative Compound identified |
References |
| Mature Gonja peel |
3.3 |
609.14 |
609.14 (15), 300.3 (15), 271.03 (2), 118.97 (15), 99.92 (46), 78.96 (100) |
Quercetin glycoside (quercetin-3-O-rutinoside)
C27H30O16 |
Sawada et al. 2009, Engels et al., 2012; Gavrilova et al., 2011) |
| 3.6 |
593.15 |
593.15 (40), 327.05 (3), 285.04 (100), 255.03 (4), 116.93 (36) |
Kaempferol glycoside (Kaempferol-3-O-rutinoside)
C27H30O15 |
Sawada et al. 2009, Engels et al., 2012; Gavrilova et al., 2011, Guimarães et al., 2013) |
| Mature Kivuvu Peel |
3.3 |
609.15 |
663.07 (1), 609.15 (88), 343.05 (1), 300.03 (100), 271.03 (10), 147.04 (9), 111.08 (20), 78.96 (79) |
Quercetin glycoside (quercetin-3-O-rutinoside)
C27H30O16 |
Sawada et al. 2009, Engels et al., 2012; Gavrilova et al., 2011) |
| Young Kivuvu Pulp |
2.1 |
341.09 |
287.04 (1), 203.08 (3), 161.02 (22), 96.96 (16), 78.96 (100) |
Caffeic- hexoside
C15H17O9 |
Sawada et al. 2009, Fu et al., (2016) |
| 3.0 |
625.14 |
625.14 (8), 316.02 (21), 271.03 (1), 96.97 (12), 78.96 (100) |
Myricetin derivative (a glucopyranoside)
C27H30O17 |
Yahia, Gutiérrez-Orozco, & Moreno-Pérez, (2017), Guimarães et al., 2013) |
| 3.2 |
739.21 |
269.05 (1), 152.90 (2), 96.97 (13), 78.96 (100) |
Apigenin glycoside C33H40O19 |
Guimarães et al., 2013) |
| 4.1 |
287.06 |
399.15 (2), 259.06 (8), 209.08 (64), 97.07 (54), 78.96 (100) |
Eriodictyol
C15H12O6 |
Lin, & Harnly, (2010). |
| 4.6 |
263.13 |
204.12 (2), 118.97 (4), 96.97 (16), 78.96 (100) |
C15H19O4 |
|
| Young Kivuvu Peel |
3.5 |
571.17 |
571.17 (100), 173.08 (18), 190.93 (10), 593.15 (12) |
N/I |
|
| 4.3 |
613.18 |
613.18 (100), 659.19 (26), 187.10 (20) |
N/I |
|
| 4.8 |
655.19 |
655.19 (16), 613.18 (4), 383.12 (1), 307.08 (1), 185.08 (1), 163.04 (10), 145.03 (100), 78.96 (42) |
N/I |
|
| 5.5 |
697.20 |
697.20 (7), 613.18 (1), 425.13 (1), 280.98 (1), 163.04 (12), 145.03 (94), 78.96 (100) |
N/I |
|
| 5.6 |
743.21 |
697.20 (16), 655.19 (3), 425.13 (1), 209.09 (4), 163.04 (11), 145.03 (100), 96.96 (22) |
N/I |
|
A study [4] also found very weak correlation between total phenolic content and FRAP activity (r2=0.1614, p < 0.0001) as well as total phenolic content and DPPH activity (r2=0.02339, p < 0.05) for the eight Malaysian bananas (Musa sp.). Results of antioxidant activity by FRAP for unripe banana flour, nanicão variety, were 3.59 μM Trolox eq/g DW [19].
The variances in the results obtained from the antioxidant activity assays used can be explained in terms of the variations in the relative quantities, forms, and reaction of the antioxidant constituents in the extracts to the colorimetric assays. According to a study [20] DPPH assay may not truly depict the total antioxidant capacity as compared to FRAP owing to its less sensitiveness towards hydrophilic antioxidants and the dependency of the interaction of antioxidant compounds with DPPH on their structural conformations.
A comparison by a study [19] of the antioxidant activity values obtained by different methods for flour from banana (Musa AAA) peel revealed that FRAP exhibited the lowest antioxidant values among the methods used i.e. ORAC, ABTS and FRAP. These researchers suggested that the compounds present in the banana peel flour act more efficiently by the mechanism of hydrogen atom transfer than electron transfer for cation radical ABTS and the ferric ion.
Phenolic compounds characterization
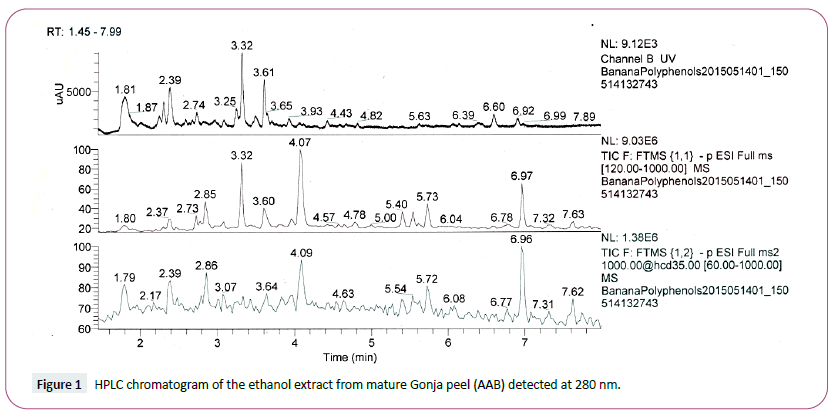
The characterization of the phenolic compounds was performed by HPLC-DAD-ESI/MS analysis and data of the retention time, pseudo molecular ion, main fragment ions in MS2 and tentative identification of phenolic compounds from mature Gonja (AAB) peel, mature Kivuvu (ABB) Peel, young Kivuvu (ABB) pulp and young Kivuvu (ABB) peel are presented in Table 2. Characterization of the phenolic compounds of these samples was based on the high total phenolic content values they exhibited. The HPLC phenolic profiles recorded at 280 nm were as shown in sample in Figure 1 and the other profiles are available in the supplementary data. The phenolic compounds were positively identified by comparing the retention times (Rt), detected mass (MS), fragment ions (MS/MS), and molecular formula with known plant metabolites in literature and databases (RRIMe and Mass bank databases) according to their retention, mass and UV–Vis characteristics by comparison in the RRIMe and Mass bank databases.
A comparison by a study [19] of the antioxidant activity values obtained by different methods for flour from banana (Musa AAA) peel revealed that FRAP exhibited the lowest antioxidant values among the methods used i.e. ORAC, ABTS and FRAP. These researchers suggested that the compounds present in the banana peel flour act more efficiently by the mechanism of hydrogen atom transfer than electron transfer for cation radical ABTS and the ferric ion.
Phenolic compounds characterization
The characterization of the phenolic compounds was performed by HPLC-DAD-ESI/MS analysis and data of the retention time, pseudo molecular ion, main fragment ions in MS2 and tentative identification of phenolic compounds from mature Gonja (AAB) peel, mature Kivuvu (ABB) Peel, young Kivuvu (ABB) pulp and young Kivuvu (ABB) peel are presented in Table 2. Characterization of the phenolic compounds of these samples was based on the high total phenolic content values they exhibited. The HPLC phenolic profiles recorded at 280 nm were as shown in sample in Figure 1 and the other profiles are available in the supplementary data. The phenolic compounds were positively identified by comparing the retention times (Rt), detected mass (MS), fragment ions (MS/MS), and molecular formula with known plant metabolites in literature and databases (RRIMe and Mass bank databases) according to their retention, mass and UV–Vis characteristics by comparison in the RRIMe and Mass bank databases.
Figure 1: HPLC chromatogram of the ethanol extract from mature Gonja peel (AAB) detected at 280 nm.
LC-MS analysis
The peak with retention time of 3.0 min had pseudo molecular ions [M-H]- at m/z 625 with the corresponding fragment ions at m/z 316 and was tentatively identified as myricetin glucopyranoside after comparison with the Yahia et al. [21] results. This myricetin glucopyranoside was only identified in young Kivuvu (ABB) pulp only.
The peak with retention time of 3.2 min had pseudo molecular ions [M-H]- at m/z 739 with the corresponding fragment ions at m/z 269 (Table 2) and was tentatively identified as an apigeninglycoside after comparison with the results from [21] This apigenin glycoside was found in young Kivuvu (ABB) pulp only.
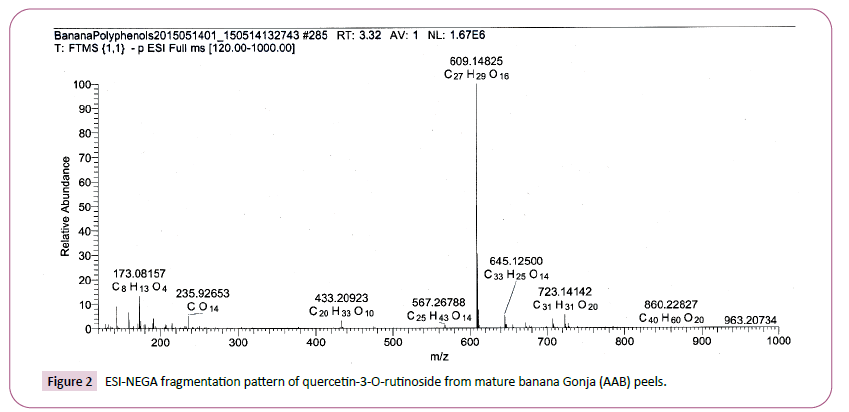
The peaks with retention time of 3.3 min had pseudo molecular ions [M-H]- at m/z 609 as well as the fragment ions at m/z 300 obtained after the removal of a rutinoside moiety [22,23]. The identity was assigned based on the pseudo-molecular ions and MS2 spectra, releasing fragments corresponding to quercetin (m/z at 301) and to distinct loss of rutinoside (-308 mu) moieties and was tentatively identified as quercetin rutinoside through reference to results [22,23] and comparison with the RRIMe database. This quercetin glycoside with retention time of 3.3 and pseudo molecular ions [M-H]- at m/z 609 was found in both mature Kivuvu (ABB) peel and mature Gonja (AAB) peels.
The peak with retention time of 3.6 min had pseudo molecular ions [M-H]- at m/z 593 as well as the fragment ions at m/z 285 a typical mass in the negative mode of a cyanidin aglycone corresponding to loss of rutinoside (m=z 308) and was found to be a Kaempferol rutinoside after comparison with literature [22,23] and the Prime database. This glycoside with retention time of 3.6 min and pseudo molecular ions [M-H]- at m/z 593 was found in mature Gonja (AAB) peel only.
The peak with retention time of 4.1 min had pseudo molecular ions [M-H]- at m/z 287 which is a typical mass of a cyanidin aglycone with the corresponding fragment ions at m/z 209 and was tentatively identified as Eriodictyol after comparison with the RRIMe database and literature [24]. Eriodictyol compound was only identified in young Kivuvu (ABB) pulp only.
The peak with retention time of 4.6 min had pseudo molecular ions [M-H]- at m/z 263 with the corresponding fragment ions at m/z 118 and was tentatively identified as C15H19O4 after comparison with the RRIMe database and was found in Young Kivuvu (ABB) pulp only.
The peak with retention time of 2.1 min had pseudo molecular ions [M-H]- at m/z 341 and was tentatively identified as Caffeic hexoside (C15H17O9) after comparison with the literature [25] and the RRIMe database and was found in young Kivuvu (ABB) pulp only.
The peaks with retention times of 3.5, 4.3, 4.8, 5.5 and 5.6 min had pseudo molecular ions [M-H]- at m/z 571, 613, 655, 697 and 743 respectively, however they were not identifiable using comparison with the RRIMe, Mass bank databases as well as in literature. All these unidentified compounds were found in young Kivuvu (ABB) peel (Figures 1 and 2).
Figure 2: ESI-NEGA fragmentation pattern of quercetin-3-O-rutinoside from mature banana Gonja (AAB) peels.
UV and mass spectra obtained by HPLC-DAD-ESI/MS analysis showed that the phenolic composition of the investigated banana varieties was characterized by the presence of flavonoids and their derivatives. The analysis of the MS2 fragments revealed glycosides mainly from Quercetin and Kaempferol. Sugar substituent’s consisted of disaccharides mainly as deduced from the loss of 308 Da.
The identified peaks at UV wavelength of 280 nm had HPLC retention times of 2.1, 3.0, 3.2, 3.3, 3.6, 4.1 and 4.6 min at 280 nm (Table 2).
Results of the polyphenolic profile of the mature Gonja peel, mature Kivuvu peel and young Kivuvu pulp and peel showed a high variability of polyphenols. Mature Gonja (AAB) peel had a high number of polyphenolic compounds identified which included phenolic acids and flavonols such as Rutin (a glycoside of quercetin) and its derivatives as well as Kaempferol derivatives (Table 2). Only one polyphenolic compound, Rutin was identified in mature Kivuvu peel while it was not possible to identify the polyphenolic compounds in the young Kivuvu peel using the available databases and literature. The identification of only one polyphenolic compound in mature Kivuvu peel indicates that only the rutin was present in quantities high enough to be identified. The results showed that the young Kivuvu pulp contains mainly Kaempferol derivatives along with quercetin derivatives and Eriodictyol. These results are in agreement with [26] who reported that some of the phenolic compounds in banana fruit included flavonolaglycones, such as myricetin, quercetin and kaempferol.
The phenolic compounds results for the peels and pulps of nine plantain cultivars and two dessert bananas as reported [33-35] showed that hydroxycinnamic derivatives like ferulic acidhexoside, were of major occurrence though highly diverse in the plantain pulp among cultivars while rutin which was identified in young Kivuvu peel was the most abundant flavonol glycoside in plantain peels. A study [10] found gallocatechin, and epicatechin in fruit pulp of postharvested ripe bananas (Musa acuminata Juss.) addition to catechin. A study [27] found ferulic acid to be the highest occurring phenolic compound in bananas with other compounds such as sinapic, salicylic, gallic, p-hydroxybenzoic, vanillic, syringic, gentisic and p-coumaric acids being present. The flour of banana peel (Musa AAA) was reported to contain, kaempferol, quercetin isorhamnetin, myricetin, laricitrin and syringetin with Quercetin-based glycosides being the most abundant flavonols, accounting for about half of total flavonols present [19].
The health promoting effects of polyphenols have often been associated with their antioxidant activity. For example, Quercetin has been found to be a very efficient antioxidant [27] and appears to play an active role in many diseases. Derivatives of this compound were found in the Mature Gonja peel, Mature Kivuvu Peel and Young Kivuvu Pulp. As far as we know, there is no information on the phenolic composition of banana fruits of the Gonja (AAB) and Kivuvu (ABB) varieties.
An investigation by a study [28] found the major phenolic compounds from the sap of banana accessions, namely, Musa balbisiana, Musa laterita, Musa ornata, and Musa acuminata, and some cultivars were apigenin glycosides, myricetin glycoside, myricetin-3-O-rutinoside, naringenin glycosides, kaempferol-3-Orutinoside, quercetin-3-O-rutinoside.
The influence of genetic and environmental factors on the composition and content of phenolic compounds is highly recognized. The study evidenced the commonly accepted trend that fruit bioactive compounds are affected by factors such as cultivar or variety and stage of maturity [29] pronounced differences in phenolic contents and composition were found in the two banana varieties tested in this study. These varietal differences may be partly attributed to the different genotypes of these banana varieties and the environmental factors could not have played a part since both banana varieties were exposed to the same environmental conditions. Similar phenomena have been reported in other fruits such as strawberry [30] and guava [31-36]. However, the other dependent factor of climate or geographic site of production was not investigated in the present study as materials were obtained from the same geographical site of production.
Results of this study represent a contribution to the chemical characterization of phenolic extracts from banana fruits of the Gonja (AAB) and Kivuvu (ABB) varieties as well as their Total phenolic content and antioxidant activities. The studied fruits may have great potential for food industries as a source of bioactive molecules such as phenolic compounds for dietary supplements or functional foods especially the peels of Gonja which are usually discarded.
Conclusion
The extracts from mainly the peels of the two banana varieties studied, were found to have the potential of being a source of high polyphenolic compounds as exhibited by the TPC results and displayed high antioxidant activities.
With the results of high phenolic content and antioxidant activity in the young bananas, this may provide some good news in Africa and the tropical regions, where natural calamities such as hail storms and wind storms destroy vast areas of banana plantations that are not yet mature rendering them useless to the owners.
With this development, the young bananas destroyed during such calamities may be utilized as sources of phenolic compounds and antioxidant activity so as to minimize the losses of the banana farmers who are predominantly peasants.
However, additional studies are required to demonstrate the practical applications for enhanced utilization of banana peels with respect to their potential as sources of functional compounds and in-depth studies into the variation of phenolic content, composition and antioxidant activity of the bananas throughout the maturity cycle is necessary to determine the most optimum point at which the highest values of above parameter are highest.
Acknowledgments
This research was funded by the Presidential Initiative on Banana Industrial Development (PIBID). The authors are grateful to Prof. Hidefumi Yoshii and the Laboratory of Food Engineering, Kagawa University, Japan for provision of the research facilities that were used in this study.
Conflicts of Interest
We hereby declare that all the authors of this manuscript have no conflict of interest pertaining to this study whatsoever.
References
- Dhamija I, Kumar N, Manjula SN, Parihar V, Setty MM (2013) Preliminary evaluation of in vitro cytotoxicity and invivoantitumor activity of PremnaherbaceaRoxb. In Ehrlich ascites carcinoma model and Dalton’s lymphoma ascites model. Experimental and Toxicological Pathology. 65: 235-242.
- Giacometti J, Muhvić D, Pavletic A, Ðudarić L (2016) Cocoa polyphenols exhibit antioxidant, anti-inflammatory, anticancerogenic, and anti-necrotic activity in carbon tetrachloride intoxicated mice. Journal ofFunctional Foods. 23: 177-187.
- Someya S, Yoshiki Y, Okubo K (2002) Antioxidant compounds from bananas (Musacavendish) Food Chem. 79 (3): 351-354.
- Sulaiman SF, Yusoff NAM, Eldeen IM, Seow EM, Sajak AAB (2011) Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.) Journal of Food Composition and Analysis. 24 (1): 1-10.
- Mèlo EA, Lima VLAG, Maciel MIS (2006) Polyphenol, ascorbic acid and total carotenoid contents in common fruits and vegetables. Brazilian Journal of Food & Technology. 9: 89-94.
- Nguyen TBT, Ketsa S, van Doorn WG (2003) Relationship between browning and the activities of polyphenol oxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biology & Technology. 30 (2): 187-193.
- Macheix JJ, Fleuriet A, Billot J (1990) Fruit Phenolics. CRC Press, Boca Raton, FL.
- Kondo S, Kittikorn M, Kanlayanarat S (2005) Preharvest antioxidant activities of tropical fruits and the effect of low temperature storage on antioxidants and jasmonates. Postharvest Biology & Technology. 36: 309-318.
- Kandasamy S, Aradhya SM (2014) Polyphenolic profile and antioxidant properties of rhizome of commercial banana cultivars grown in India. Food Bioscience. 8: 22-32.
- Bennett RN, Shiga TM, Hassimotto NMA, Rosa EAS, Lajolo FM, et al. (2010) Phenolics and antioxidant properties of fruit pulp and cell wall fractions of postharvest banana (musa acuminata juss.) Cultivars. Journal of Agricultural and Food Chemistry. 58 (13): 7991-8003.
- Kawongolo JB (2013) Optimization of Processing Technology for Commercial Drying of Bananas (Matooke) PhD Thesis. University of Kassel.
- Sentandreu E, Cerdán-Calero M, Navarro JL (2015) Metabolite profiling of pigments from acid-hydrolysed persimmon (Diospyros kaki) extracts by HPLC-DAD/ESI-MSn analysis. Journal of Food Composition & Analysis. 3855-61.
- Singleton VL, Rossi JA, Jr (1965) American J. of Enol. Vitic. 16-144.
- Blois MS (2002) Antioxidant determinations by the use of a stable free radical. Nature. 26: 1199-1200.
- Sawada Y, Akiyama K, Sakata A, Kuwahara A, Otsuki H, et al. (2009) Widely targeted metabolomics based on large-scale MS/M data for elucidating metabolite accumulation patterns in plants. Plant cell Physiology. 50: 37-47.
- Fatemeh SR, Saifullah R, Abbas FMA, Azhar ME (2012) Total phenolics, flavonoids and antioxidant activity of banana pulp and peel flours: Influence of variety and stage of ripeness. International Food Research Journal. 19 (3): 1041-1046.
- González-Montelongo R, Lobo MG, González M (2010) Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chemistry. 119: 1030-1039.
- Babbar N, Oberoi HS, Uppal DS, Patil RT (2011) Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Research International. 44: 391-396.
- Rebello LPG, Ramos AM, Pertuzatti PB, Barcia MT, Castillo-Muñoz N, Hermosín-Gutiérrez I (2014) Flour of banana (Musa AAA) peel as a source of antioxidant phenolic compounds. Food Research International. 55: 397-403.
- Kaur C, Kapoor HC, 2001. Antioxidant in fruits and vegetable-the millennium’s health. International Journal ofFood Science and Technology. 36: 703-725.
- Guimarães R, Barros L, Dueñas M, Carvalho AM, Queiroz MJRP, et al. (2013) Characterization of phenolic compounds in wild fruits from Northeastern Portugal. Food Chemistry. 141 (4): 3721-3730.
- Engels C, Grater D, Esquivel P, Jimenez VM, Ganzle MG, et al. (2012) Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra-high- performance liquid chromatography/electrospray ionization mass spectrometry. Food Research International. 46: 557-562.
- Gavrilova V, Kajdzanoska M, Gjamovski V, Stefova M (2011) Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. Journal of Agricultural & Food Chemistry. 59: 4009-4018.
- Lin LZ, Harnly JM (2010) Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat) Food Chemistry. 120 (1): 319-326.
- Fu ZTuZ, Zhang L, Wang H, Wen Q, Huang T (2016) Food Bioscience Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Bioscience. 15: 11-18.
- Kevers C, Falkowski M, Tabart J, Defraigne JO, Dommes J, et al. (2007) Evolution of antioxidant capacity during storage of selected fruits and vegetables. Journal of Agriculture & Food Chemistry. 55: 8596-8603.
- Russell WR, Labat A, Scobbie L, Duncan GJ, Duthie GG (2009) Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chemistry. 115: 100-104.
- Pothavorn P, Kitdamrongsont K, Swangpol S, Wongniam S, Atawongsa K, et al. (2010) Sap phytochemical compositions of some bananas in Thailand. Journal of Agricultural and Food Chemistry. 58 (15): 8782-8787.
- Scalbert C, Manach C, Morand C, Remesy C (2005) Dietary polyphenols and prevention of diseases. Critical Reviews in Food Science & Nutrition. 45: 287-306.
- Meyers KJ, Watkins CB, Pritts MP, Liu RH (2003) Antioxidant and antiproliferative activities of strawberries. Journal of Agricultural & Food Chemistry. 51: 6887-6892.
- Ajila CM, Brar SK, Verma M, Tyagi RD, Valéro JR (2011) Solid-state fermentation of apple pomace using Phanerocheate hrysosporium - liberation and extraction of phenolic antioxidants. Food Chemistry. 126: 1071−1080.
- Benzie IFF, Strain JJ (1999) Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology. 299: 15-27.
- https://www.worldlistmania.com/worlds-largest-bananas-producing-countries/Accessed on 7th June 2019.
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition & Analysis. 19: 669-675.
- Tsamo CVP, Herent M, Tomekpe K, Emaga TH, Quetin-Leclercq J, et al. (2015) Phenolic profiling in the pulp and peel of nine plantain cultivars (Musa sp.) Food Chemistry. 167: 197-204.
- Zhang L, Tu ZC, Wang H, Fu ZF, Wen QH (2015) Comparison of different methods for extracting polyphenols from Ipomoea batatas leaves, and identification of antioxidant constituents by HPLC-QTOF- MS2. Food Research International. 70: 101-109.