Review Article - (2016) Volume 2, Issue 1
Sunishtha Singh Yadav1, Manish Kumar Singh2 and Rajesh Singh Yadav3*
1Amity Institute of Biotechnology, Amity University, Noida - 201 301 (UP), India
2Department of Pharmacology, King George Medical University, Lucknow 226 003, UP, India
3Department of Criminology and Forensic Science, School of Applied Sciences, Dr. Harisingh Gour Central University, Sagar - 470 003 (MP), India
*Corresponding Author:
Rajesh Singh Yadav
Department of Criminology and Forensic Science, School of Applied Sciences
Dr. Harisingh Gour Central University
Sagar – 470 003 (MP), India
Tel: +91-7582-264122
Fax: +91-7582-264163
E-mail: razitrc@gmail.com
Received date: December 28, 2015; Accepted date: January 13, 2016; Published date: January 22, 2016
Citation: Yadav SS, Singh MK, Yadav RS. Organophosphates Induced Alzheimer’s Disease: An Epigenetic Aspect. J Clin Epigenet. 2016, 2:1. DOI: 10.21767/2472-1158.100010
Copyright: © 2016 Yadav RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Alzheimer's disease is insidious progressive age related neurological disorder which leads to the loss of cognitive functions, memory processes and associated behavior. The role of environmental factor and genetic susceptibility in the etiology of Alzheimer's disease has been reviewed in recent years. Organophosphates, a class of insecticide are widely used in the agriculture, home, garden, public health programs and therefore associated with the risk of developing Alzheimer's disease in exposed population. Frequent exposure of organophosphates to human populations especially in developing countries has generated insightful concerns among the health scientists about their neurotoxic consequences. The exposure is quite imminent as most of the people are unaware of its toxic insults and handle these chemicals without the safety measures. The exposure may also occur through the consumption of contaminated food products and environmental toxicity. Recent studies suggested that exposure to organophosphates has also been linked with the neurological disorders including Alzheimer's disease. The involvement of various molecular and neurochemical signatures in its neurotoxicity are reported but the specific biomarkers, targeted mechanism of action and epidemiological link between the organophosphate exposure and pathophysiology of Alzheimer's disease are not clearly understood. The present review has therefore been aimed to add new information to the literature which may help to find out protective and preventive strategies against their neurotoxicity and may establish a possible link of organophosphate exposure and Alzheimer's disease.
Keywords
Organophosphates; Cholinergic deficits; Alzheimer’s disease; Mitochondrial dysfunctions; Apoptosis
Introduction
The lack of full proof therapy for neurodegenerative disorders and CNS diseases resulted in an increase negative impact on the quality of life and become an economic burden onto the society. Alzheimer’s disease is a progressive neurodegenerative disorder associated with the loss of cholinergic neurons and the presence of excessive neuritic plaques containing amyloid β protein and abnormal tau protein filaments in the form of neurofibrillary tangles [1,2]. Decreased level of acetylcholine in Alzheimer’s disease patients appears to be a critical element in producing dementia and memory disorders [3]. Cholinergic neurodegeneration have also been found to be associated with the loss of acetylcholine [4]. The disease includes multiple pathophysiological factors, including flawed amyloid beta protein metabolism, abnormalities of glutamatergic, adrenergic, serotonergic and dopaminergic neurotransmission and also the involvement of inflammatory cytokines [1,5]. Although the Alzheimer’s disease is an age related progressive neurological disorder, early onset of disease in the 40s and 50s has also been reported in people [6]. Several factors such as environmental exposure and genetic predisposition could be involve [7,8] in reducing the synthesis of acetylcholine, deposition of amyloid beta [9] alterations in tau protein in the brain [10], oxidative stress and inflammatory cytokines [11]. The disease is rapidly progressive, leading to the loss of cognitive functions, especially memory processes and associated behavior. The precise cause of Alzheimer’s disease is not entirely known, but the role of genetic factors, including amyloid precursor protein (APP), presenilin 1, presenilin 2 and apolipoprotein E gene has been found to be associated with the pathophysiology of Alzheimer’s disease [12]. The prevalence of Alzheimer’s disease due to genetic susceptibility in the pesticide exposed population becomes a serious matter of concern in recent years [5,13].
The production and consumption of pesticides have increased many folds after the Green Revolution in India and many other parts of the globe. Most of these pesticides are neurotoxic in nature; their direct or indirect exposure to human may lead to the neurological deformities and disorders. Of various insecticides, organophosphates are frequently used in agriculture, homes and public health programme to control pests and vectors and in veterinary practices to control ectoparasites, hence distributed in the environment [14,15]. Human exposure to these organophosphates is quite imminent due to the indiscriminate and excessive use in occupational and non-occupational settings [16,17] which may associate with various neurodegenerative diseases [5,18,19]. Cases of organophosphate poisoning from India and many other countries have been frequently reported [15,20]. Also, these organophosphate compounds are expected to involve in the highest incidences of suicidal poisoning in India [15,21]. High levels of residues of organophosphate and their metabolites detected in the dietary products and biological tissues of exposed individuals are again a matter of concern due to associated toxic health effects [22-24]. The possible role of pesticide exposure, especially organophosphates in Alzheimer’s disease and dementia has not received large attention and therefore its consequences occur in the society [25]. The present review has therefore been focused on organophosphates induced mitochondrial dysfunctions, apoptotic signaling, amyloid processing, β-amyloid segregation and impairment of synaptic transmission to establish a possible epigenetic link between the pesticide exposure and pathophysiology of Alzheimer’s disease and clinical implications.
Organophosphates Induced Neurotoxicity
The possible association of chronic pesticide exposure with the increased prevalence of dementia and Alzheimer’s disease has been reported [13,26]. Organophosphates severely damage the brain and affect the behavioral pattern and neurological activities in exposed individuals [27-29]. The specific pattern of damage is cholinergic dysfunctions associated with learning and cognitive deficits, impaired neurobehavioral and neuropsychological performance [20]. Human exposure to organophosphate including monocrotophos, chlorpyrifos and dichlorvos is extensively reported due to their wide applications and presence as a contaminant in the dietary and food products [15,22]. Study on farmers in Egypt using organophosphates, including monocrotophos reported that 50% of the workers had neurological symptoms such as loss of reflexes [30]. The workers, including applicators, technicians, and engineers working in Egyptian cotton production have been found to have a substantially higher degree of chlorpyrifos exposure associated with neurobehavioral deficits [31]. They further demonstrated a dose effect relationship between urinary trichloro-2-pyridinol (TCPy), a biomarker for chlorpyrifos exposure and both plasma butyl cholinesterase (BChE) and red blood cell AChE in chlorpyrifos exposed workers [17]. In another study, [32] reported that chlorpyrifos can result in persistent alterations in axonal transport in the living mammalian brain which may lead to neurological deficits in humans repeatedly exposed to organophosphates. [33], in his study suggested that exposure through different organophosphorus pesticides could lead to the cognitive, psychomotor and emotional disturbances in individuals. The presence of residues of organophosphates, including chlorpyrifos and its metabolites in maternal prenatal and postnatal blood, cord blood and in maternal and child urine has suggested the developmental neurotoxicity of organophosphates [34-36]. In a recent study, [37] reported that exposure to phosphomedon in rats caused neurobehavioral abnormalities such as reduce food intake, weight loss, increase water intake, low defecation frequency and altered locomotion frequency. At the same time a severe histopathological changes were also observed, which was found to be associated with the neurobehavioral changes suggesting the neuronal loss. In the environment, the risk of exposure through multiple chemicals at a single time may cause their synergistic effects. In this connection a study on the exposure of real life doses of malathion, DEET and permethrin, alone or in combination in rats have been reported to cause significant neurobehavioral deficits and neuronal degeneration [38]. Repeated dose of malathion and diazinon in rats has been found to cause oxidative stress, inhibit brain and plasma cholinesterase cause, histopathological and immune alterations in brain and other body organs [39]. Recently, [40] showed that combined exposure of chlorpyrifos and lead acetate reduce the activities of brain antioxidant enzymes and AChE and increased lipid peroxidation. The changes were further linked to the altered histopathological structure of the cerebral cortex in rats [41] found that diazinon and its oxygen metabolite diazoxon increases oxidative stress in astrocytes and adversely affect astrocyte function, resulting in inhibited neurite outgrowth in hippocampal neurons linked to the decreased levels of astrocytic fibronectin.
Organophosphate Induced Oxidative Stress and Alzheimer's Disease
Organophosphates induced free radical generation linked with enhanced oxidative stress in humans has been suggested as one of the key mechanism of their neurotoxic alterations [42,43]. The amyloid β protein is found to be an important factor to enhance oxidative stress linked with increased levels of lipid peroxidation products including malondialdehyde, 4-hydroxynonenal (HNE) and acrolein [44,45]. These toxic products, formed as a result of oxidative stress alter the cellular structure and physiological function of the brain and leads to neurodegenerative diseases, including Alzheimer's disease [46,47]. The involvement of lipids, inflammatory mediators in the production and accumulation of β-amyloid and enhanced oxidative stress in Alzheimer’s disease has also been reported [48-50]. We have also reviewed and suggested that the generation of reactive oxygen and nitrogen species as a result of pesticide exposure could damage the lipid membrane and alter the composition of lipid rafts leading to various brain related disorders [51]. On the other hand the accumulation of transition metals including iron further involve in the generation of free radicals through the process of Fenton’s reaction. Docosahexenoic (DHA), dietary essential polyunsaturated fatty acids (PUFA), another target in oxidative damage has been found to link with the cognitive decline and neuronal dysfunction in Alzheimer's disease [52]. The alterations in the brain lipid profile, including phospholipids, sphingomylein, ceramide and ganglioside could modulate the signaling cascade and neural function, leading to neurological disorders, including Alzheimer’s disease [53,54]. Further altered levels of sphingomyelins andceramides in Alzheimer’s disease brains have been reported as a result of sphingomyelin hydrolysis [55].
Organophosphate Induced Neuronal Loss via Apoptosis
Acetyl cholinesterase (AChE), an enzyme involved in the synaptic transmission is the prime target of action of organophosphates. They inhibit the activity of AChE in an irreversible manner and caused over accumulation of the levels of acetylcholine at the synaptic junction leading to desensitization of receptors and finally paralysis and cell death [49,56]. Mitochondrial dysfunction and oxidative metabolism are considered to be the key mechanism for organophosphates induced apoptosis and in the pathogenesis of Alzheimer's disease [57]. Mitochondria play a vital role in apoptotic pathways, as it contains decisive apoptotic factors, including cytochrome C in their intermembranous space [58]. Once the cytochrome C release into the cytosol, it initiates the activation of caspase-cascade mechanisms of apoptosis [59]. The anti-apoptotic protein family, such as Bcl- 2 and Bcl-xL strictly regulate the release of cytochrome C and maintain the ratio between Bcl2/Bax [60,61]. The decrease in the ratio of Bcl2/Bax due to oxidative stress initiates the release of cytochrome C and activation of caspase-cascade and leads to the apoptosis [62]. The activation of caspases provides a crucial factor in the implementation of mitochondria mediated apoptosis [63]. Enhanced oxidative stress following exposure to monocrotophos in rats has been found to affect mitochondrial complex I, II and IV associated with decreased production of ATP [64]. The over activation of apoptotic factors in central nervous system can contribute to the neuronal cell death and may cause neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases [65,66]. Several studies have suggested that the toxicological response of organophosphates and its compounds may cause neuronal apoptosis linked with organophosphate induced delayed neuropathy (OPIDN) [58,67].
The alteration in lipid rafts composition may also initiate the neurodegeneration and apoptosis through various pathways including aggregation of amyloid beta. Increased expression of apoptotic proteins including Bax, JNK, c-jun, ERK1/2, MAP kinases and decreased expression of anti-apoptotic proteins such as p38 MAP kinase, Bcl-2, Bcl-xL have been reported in the organophosphate induced neurotoxicity [60,61,68] reported that exposure to chlorpyrifos may induce apoptosis in primary cortical neurons cultured from embryonic day 17 or newborn rats independently of AChE inhibition. They further suggested that the activation of the ERK1/2 and JNK MAP kinases involve in apoptotic and activation of the p38 MAP kinase in antiapoptotic mechanism in cortical neurons following exposure to chlorpyrifos. Exposure to organophosphates enhanced the levels of intracellular calcium, which triggered the activation of calpains in nerve tissues [69]. This activated calpains may further activate the cyclin-dependent kinase 5 (Cdk5) and involved in the neuronal cell death [59,70]. The detailed mechanism of neuronal apoptosis is illustrated in the Figure 1. Exposure to organophosphates, including monocrotophos, dichlorvos, chlorfenvinphos, chlorpyrifos, malathion, quinalphos etc. have been found to disrupt the balance of antioxidant and pro-oxidant in the brain and linked with enhanced oxidative stress [51,71]. Increased lipid peroxidation in brain regions and cerebro-spinal fluid of rats has been reported following exposure to malathion [72]. Exposure of triazophos in rats has been found to cause increased lipid peroxidation associated with decreased mRNA and protein expression of brain derived neurotrophic factor (BDNF) and reduced glutathione in hippocampus suggesting the role of oxidative stress in the toxicity of triazophos [73]. Further evidences also indicated that the generation of reactive nitrogen species through activated astrocytes and oxidative stress are involved in various neurodegenerative diseases, including Alzheimer’s disease, ischemia, epilepsy etc. [74-76].
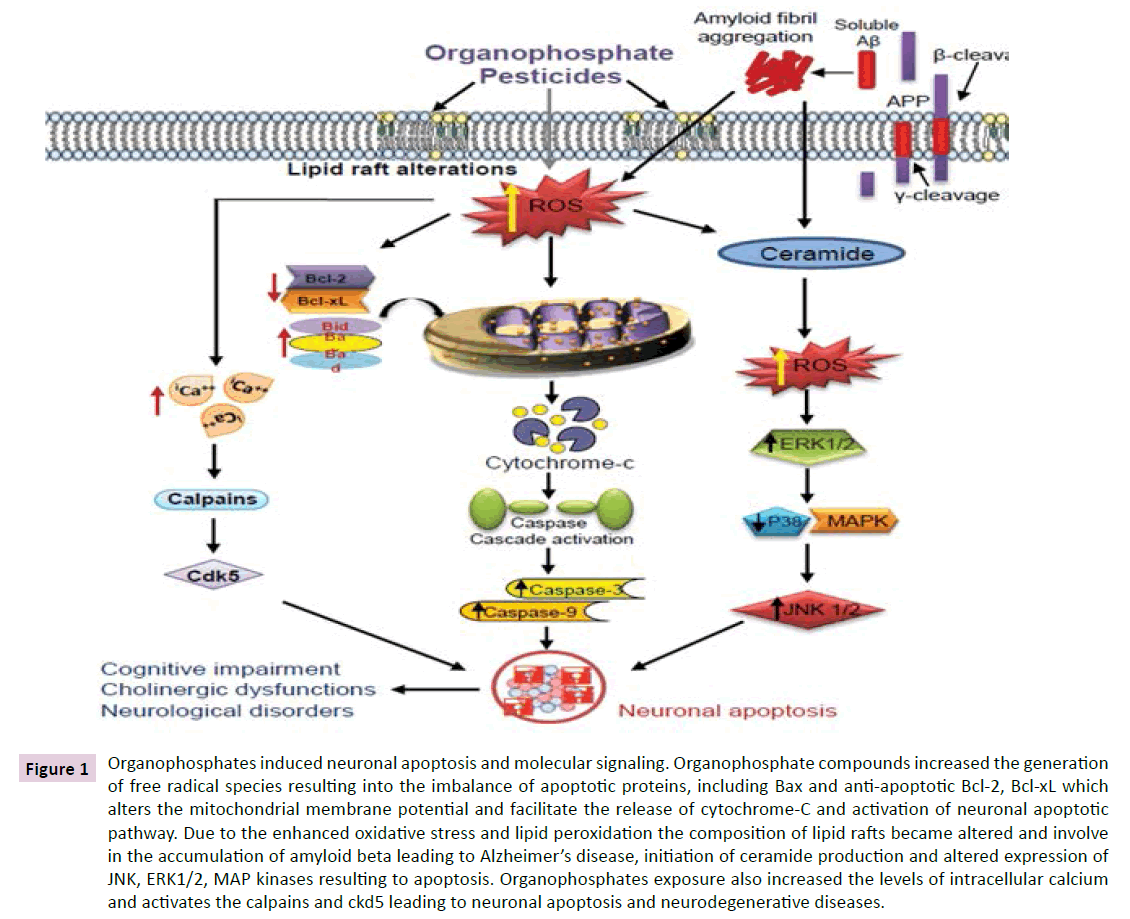
Figure 1 Organophosphates induced neuronal apoptosis and molecular signaling. Organophosphate compounds increased the generation of free radical species resulting into the imbalance of apoptotic proteins, including Bax and anti-apoptotic Bcl-2, Bcl-xL which alters the mitochondrial membrane potential and facilitate the release of cytochrome-C and activation of neuronal apoptotic pathway. Due to the enhanced oxidative stress and lipid peroxidation the composition of lipid rafts became altered and involve in the accumulation of amyloid beta leading to Alzheimer’s disease, initiation of ceramide production and altered expression of JNK, ERK1/2, MAP kinases resulting to apoptosis. Organophosphates exposure also increased the levels of intracellular calcium and activates the calpains and ckd5 leading to neuronal apoptosis and neurodegenerative diseases.
Organophosphate induced Inflammation and Alzheimer’s disease
The role of inflammation in the etiology and pathogenesis of Alzheimer’s disease has been suggested [77,78]. The activation of these inflammatory cytokines occurs due to the enhanced oxidative stress, which may involve in the process of neurodegeneration in Alzheimer’s diseases [79]. The role of microglia in Alzheimer’s disease has been suggested due to the presence of plaque associated microglia that exhibits a reactive phenotype [77,80]. The inflammatory response is primarily expressed by the activation of glial cells, macrophages and oligodendrocytes in the brain associated with the triggering of pro-inflammatory cytokines including interleukin (IL)-1β, IL-18 and IL-33 and linked with the infection, autoimmunity, neuroinflammation and associated disorders [81-83]. These activated microglias are involved in the process of apoptosis and neuronal death via the secretion of various proinflammatory molecules and cytokines (IL-1, IL-6 and TNF-α) and also facilitate the production and deposition of amyloid in the brain [77,84,85] have demonstrated that dichlorvos exposure in rats can activate microglial cells and cause apoptosis through the upregulation of pro-inflammatory molecules like nitric oxide, TNF-α, and IL-1β. The microglial apoptosis has also been found to be associated with the increased expression of Bax in mitochondria, cytochrome c release from mitochondria, and caspase-3 activation. The role of inflammasomes in the pathophysiology of neuroinflammation and neurodegenerative diseases including dementia, memory and cognitive dysfunctions has been reported in the last decades [78,83,86]. Also the role of inflammasomes in the etiologies of Alzheimer’s disease has been suggested [87]. The roles of DNA methylation and hydroxymethylation in the development and potential treatment of AD have also been reported [88,89] in their review has suggested that oxidative stress, neuroinflammation, microtubule alterations, synthesis of beta amyloid, calcium dyshomeostasis and mitochondrial dysfunction all are contributing factors in organophosphate induced neurological diseases.
Prevention and Suggestions
The risk of human exposure to organophosphates is enhanced several times in developing countries due to the irregularities in safety measures. Due to the high risk of neurotoxic impact of these organophosphate compounds on human health, especially on developing children, it deserves the attention of regulatory agencies and prevention authorities. There is a need to develop the protective measures for agricultural workers and other individuals occupationally exposed to these pesticides. The industrial manufacturers should have to use proper safety measures and also aware the workers, users and general public about their harmful consequences through improper handling and uses. There should be trainings and workshops on these pesticides, including organophosphates to awareness and educate the users in agricultural sectors and public health programs. The research should be continued in the area of developing substitute of these toxic compounds for their use in agriculture and public health. Specific biomarkers could be identified in the study of molecular mechanisms of neurotoxicity of organophosphate compounds through parallel studies in humans and animals which could help to develop a protective and effective cure [90]. At the same time these biomarkers may also provide strategies to identify the risk in exposed individuals. In recent years natural and pharmacological agents have been found to combat the neurotoxic effects of organophosphate compounds [91-95]. Further, to assess the neurotoxic impact of specific pesticides in human populations, there is a need to perform well designed epidemiological studies which could provide useful information to research scientist working in the area of occupational, environmental and human health. Also, animal and alternate animal model research must be going on to find out the mechanisms of neurotoxicity of these pesticides at low dose levels comparable with real world exposure. Invasive research on molecular basis may improve the understanding of mechanism of neurotoxicity from organophosphate exposure and hence will be useful to develop protective measures.
Conclusion
The use of pesticides, especially organophosphates and its associated neurochemical alterations and neurological disorders in both adults and children become a serious concern among the health scientists to use protective and preventive approaches for minimizing its neurotoxicity. Some of the organophosphates are banned in India and in several other countries, but their injudicious use is further a matter of concern world over. The present review may help to understand the detailed mechanism of organophosphate induced neurotoxicity and to find out preventive measures accordingly. The study may provide new insights into neurotoxicity and open new vistas for regulatory agencies to use the human data in risk assessments of pesticides. In the present study, an attempt has been made to add new information to the literature regarding the overall neurotoxicity and the general mechanisms of toxic actions of organophosphate pesticides. A detailed understanding of the molecular and cellular toxic events linked with signaling cascade in the brain is required for effective cure, prevention and management of organophosphate induced neurotoxicity.
Conflict of Interest
There is no conflict of interest.
Acknowledgement
The authors are thankful to Dr. Harisingh Gour Central University, Sagar (MP), India for providing the opportunity to work and their support and interest. Dr. Rajesh Singh Yadav is also thankful to the University Grants Commission (UGC), New Delhi, India for providing the UGC-BSR startup research grants.