Research Article - (2023) Volume 7, Issue 1
Histopathological Effect of Ranitidine (Zantac) on Liver and Kidneys in Albino Mice
Muna Salah Rashid*
Department of Biology, University of Tikrit, Salah Al-deen, Iraq
*Correspondence:
Muna Salah Rashid,
Department of Biology, University of Tikrit, Salah Al-deen,
Iraq,
Tel: 009647706647061,
Email:
Received: 02-Jan-2023, Manuscript No. IPJCGH-23-15631;
Editor assigned: 04-Jan-2023, Pre QC No. IPJCGH-23-15631 (PQ);
Reviewed: 18-Jan-2023, QC No. IPJCGH-23-15631;
Revised: 23-Jan-2023, Manuscript No. IPJCGH-23-15631 (R);
Published:
30-Jan-2023, DOI: 10.36648/2575-7733.7.1.1
Abstract
The Purpose: This study was sought to determine the effect of ranitidine on the histological structure of liver and kidneys in the albino mice.
The Design: Two groups of mice were administered Ranitidine 75 mg/kg of and 150 mg/kg respectively, we used (15) albino mice and administrated the doses orally for 10 consecutive 10 days.
Finding: The results showed some structural effects on tissues of liver and kidneys group two showed (75 mg/kg of b.w.) desquamation of the wall of central vein of the liver, necrosis, accumulation of fibroblast, hemorrhage and aggregation of Kupffer cells the kidneys exhibited necrosis in some regions, hemorrhage and existence of cast in the tubules and it seems that ranitidine has caused dilation between glomerulus and bowman corpuscles. Group three (150 mg/kg of b.w.) revealed more damage in the wall of the central vein of the liver, infiltration of focal inflammatory in the area caused hemorrhage, expansion in sinusoid, accumulation of fibroblast and necrosis in the liver tissue. Kidneys were severely affected in comparison to group one, and showed an extended hemorrhage and ruptures in glomerulus with dilatation, hyperplasia cells in tubules and cast, form this study we conclude that ranitidine with a dose of 75 mg/kg has mild effect on the liver and kidneys and medium effect with a dose of with a dose of 150 mg/ kg.
Originality/Value: The research is focus in histopathology effects of ranitidine on liver and kidneys.
Keywords
Ranitidine; Liver; Kidneys; Necrosis; Fibroblast
Introduction
In April 2020, the FDA decided to withdraw all counter ranitidine
(zantac) drug from pharmacies after claims that it contain
certain levels of N-nitrodimethylamine (NDMA) [1]. NDMA,
a potential carcinogen, is a natural substance, occur in small
amounts in bodies, processed foods and treated water. Although
several medications that contain NDMA are still available,
ranitidine has been immediately withdrawn from the
shelves. Later on, it has been found that the levels of NDMA in
ranitidine increased over time [2].
Therefore, many researchers were sought to study and analyze
Ranitidine components using high throughput analytical technologies. Investigations revealed that the elevated level of
NDMA was attributed to the degradation of Ranitidine molecules
over time due to improper storage during hot and humid
seasons and exposure to disinfectants found in drinking water
(Figure 1) [3].
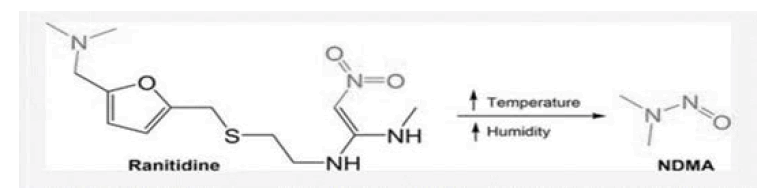
Figure 1: Showing the slow degradation of ranitidine and form of NDMA by heat and humidity
After low amounts of the possible human carcinogen N-nitrosodimethylamine
(NDMA) were detected in certain batches of
over-the-counter ranitidine tablets (75 mg and 150 mg), the US
Food and Drug Administration has asked patients and doctors
to return drugs [4]. The FDA advance information to the public
and doctors, declaring that some ranitidine medications may
comprise low levels of NDMA and requesting that any tablets labelled by Walgreens, Walmart, or Rite-Aid and manufactured
by Apotex Corporation be returned, as well as recalling 14 lots of medication ranitidine capsules spread by Sandoz, Novartis’
generic division, for the same reason [5].
NDMA is a recognized human carcinogen that has the potential
to cause cancer. It’s a well-known pollutant that can be found
in water and foods such as meat, dairy, and vegetables [6].
Clinical examination of side effects of Zantac are very similar
to the symptoms recorded by placebo, according to French reports,
The most common side effects are: Clay-colored stools,
dark urine, loss of appetite, coughing with mucous, increase
heartbeat, weakness, vertigo and dizziness, also can cause liver
failure and kidney failure [1]. Due to these factors, the aim of
this research was to determine the effect of ranitidine in the
histological.
Materials and Methods
Animals
Fifteen male albino mice (Mus musculus albinus), 5 weeks of
age, weighing 25 g ± 3 g were purchased from the animal house
of college of Veterinary Medicine, University of Tikrit, Iraq. The
mice were kept for 7 days in cages under controlled conditions
of temperature (25°C ± 2°C) and light (12 hours/12 hours) at
the university animal house and were given standard food pellets
and water. All animals were used in the experiment.
Drug preparation: Ranitidine tablets were purchased from local
pharmacies as ZantacR hydrochloride 75 mg/kg b.w. and
150 mg/kg b.w. Tablets were crushed without any further purification,
dissolved in normal saline and administered to the
animals orally with the given dosages.
Experimental Design
Animals were divided into three groups 5 mice each, group one
is the control group and administered distilled water orally for
10 days. Groups 2 and 3 were the treatment groups and administered
57 mg/kg and 150 mg/kg b.w. normal saline suspension
of ranitidine orally for 10 days. Animals were subjected to
daily monitoring and weights were taken before and after the
experiment. At the end of the experiment, the animals were
anesthetized, dissected and the liver and kidneys were stored
with formalin 10% for the subsequent experiment.
Tissues Preparation
Tissues were performed according to Suvarna, et al. (2019)
briefly, all sections were fixes with formalin 10%, and preparation
dehydrated once with ethanol 50%, 70%, 90%, 96% and
twice with 100% for 30 minutes each concentration [7]. Afterward,
sections were cleared with xylol, infiltrated and embed embedded
in paraffin wax. Subsequently tissues were subjected to
sectioning by rotary microtome (Microtec-Rotary Microtome
Cut4060-Germany), and stained with hematoxylin and eosin.
Finally, slides were made, mounted by DPX, examined by light
microscope (OBL-137C832DIGITAL MICROSCOPE SET 4x-100x
with 3W LED (transmitted light), with Tablet camera 5mp,
WLAN, USB 2.0, HDMI, SD,CMOS 1/2,5” inclusive of C-MOUNT
ADAPTER), and processed by Photoshop (Adobe Photoshop CC
2021, USA).
Results and Discussion
Effect of Ranitidine on the Liver
In group one, control group, the liver has normal, the central
vein and the hepatocytes were normally arranged as a cord
regulated around the central vein, the sinusoid between the
hepatocytes cords and could see the Kupffer cells in the sinusoids
(Figure 2A). In group two that has been treated orally
with Ranitidine 75 mg/kg, the liver have been mildly affected.
Liver showed necrosis in some areas, desquamation of the wall
of central vein, aggregations of fibroblasts, hemorrhage due to
desquamation; hepatocytes were normally arranged with the
presence of Kupffer cells (Figure 2B). Group two, where animals
treated with Ranitidine 150 mg/kg showed more histopathological
changes compared to group two. Sever damage in
the wall of the central vein, focal inflammatory cells infiltration
near the central vein resulting in hemorrhage, expansion in sinusoid.
Moreover, more sever necrotic foci in some regions and
accumulation of high number of fibroblasts (Figure 2C). Table 1 shows the measure of central vein an hepatocytes the measure
of central vein is deceased with the high concentration but the
hepatocytes is smaller with the high concentration.
| g |
label |
area |
mean |
Std Dev |
| control |
central vein |
9065.156 |
201.747 |
68.53 |
| control |
hepatocyte |
5892.351 |
166.209 |
18.122 |
| Group 1 |
central vein |
13500.7 |
218.803 |
11.406 |
| Group 1 |
hepatocyte |
5167.139 |
163.678 |
14.757 |
| Group 2 |
central vein |
16951.84 |
229.261 |
7.255 |
| Group 2 |
hepatocyte |
3716.714 |
157.935 |
12.389 |
Table 1: Shows the measurement of central vein and hepatocytes
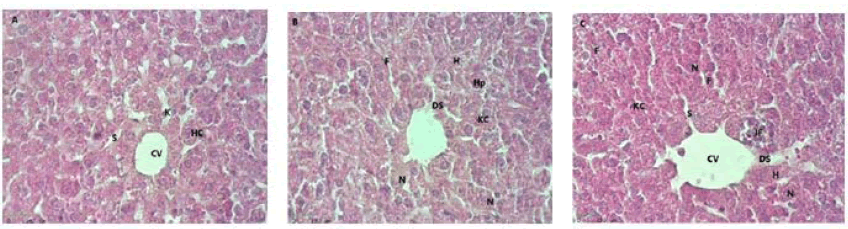
Figure 2: Shows the effect of Ranitidine on mice liver. (A) The control group liver shows normal central vein (CV), normal hepatocytes (Hp), normal sinusoid (S), presence of Kupffer cells (KC), (B) Liver of mice treated with (75 mg/kg/b.w.) Ranitidine showing desquamation of the wall of central vein (DS), necrosis (N), aggregation of fibroblasts (F), hemorrhage (H), normal hepatocytes (Hp), presence of Kupffer cells (KC) and (C) Liver of mice treated with (150 mg/kg/b.w.) Ranitidine showing damage in the wall of the central vein (DS), focal inflammatory cells infiltration near the central vein (IF) causing hemorrhage (H), expansion of sinusoids (S), necrosis in some regions (N) and aggregation of fibroblasts (F), (H and E) 400X.
Effect of Ranitidine on Kidneys
In the control group the kidneys have normal bowman corpuscles
glomerulus, and tubules (Figure 3A). In group two where mice treated orally with 75 mg/kg of Ranitidine, kidneys
showed large space between glomerulus and bowman corpuscle,
hemorrhage, necrosis in some region and presence of casts
in the tubules (Figure 3B). In group three, where mice treated
orally with 150 mg/kg ranitidine, kidneys were severely affected
compared with second group. There were rupturing and increased
of hemorrhage in glomeruli and large space of the tissue,
hyperplasia cells in tubules and cast (Figure 3C). (Table 2)
the measured of glumerula and tubules show the decrease of
the glumerula in the second group (group 1) and be in normal
measure in third group (group 2), tubules be in large size in the
second group (group 1) and be normal in third group (group 2).
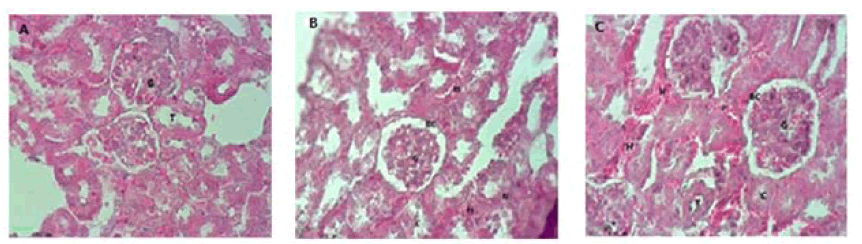
Figure 3: Shows the effect of Ranitidine on mice kidneys. (A) Normal bowman corpuscles and glomerulus (G) and normal tubules (T). (B) Kidney of mice treated with (75 mg/kg) Ranitidine showing large space between glomerulus (G) and bowman corpuscle (BC), Hemorrhage (H), necrosis (N) cast in the tubules (C) and (C) kidney of mice treated with (150 mg/kg) Ranitidine showing rupturing in glomeruli (G), hemorrhage (H) and hyperplasia cells in tubules (T), and casts (C), (H and E) 400X.
| g |
label |
area |
mean |
Std Dev |
| control |
glumerula |
10556.7 |
165.414 |
29.683 |
| control |
tubules |
7351.575 |
175.295 |
28.195 |
| Group 1 |
glumerula |
11247.17 |
135.359 |
41.11 |
| Group 1 |
tubules |
5287.46 |
197.15 |
32.641 |
| Group 2 |
glumerula |
12879.82 |
150.196 |
33.356 |
| Group 2 |
tubules |
5347.222 |
175.703 |
30.154 |
Table 2: The measurement of glumerula and tubule
The results corresponded with those of who employed Ranitidine
administration in male rats at doses of 10 mg/kg, 30 mg/
kg, and 50 mg/kg b.w. for three weeks [8]. They indicated negative
effects in rats given 10 mg/kg and 30 mg/kg. Treatment
with 50 mg/kg resulted in a significant rise in the activity of
acid phosphatase in the liver and aspartate aminotransferase
(AST) in the serum and liver, as well as a tendency for an elevation
of serum alanine aminotransferase (ALT). There was also a
significant decrease in serum activity of both amylase and alkaline
phosphatase. Microscopic analysis of same animals’ liver
tissue revealed a lack of certain hepatic cells, pyknotic nuclei, blood sinusoids expansion, binucleated cells, and lymphocyte
infiltration.
In vitro and in vivo, there are significant correlation in ranitidine
accumulation and ADME characteristics; oral ranitidine
has a medium absorption. Microsomal enzymes catalyze hepatic
metabolism, which plays a modest part in total clearance
[9]. Kidney disorders In humans, around 30% of the oral dose
is excreted as unaltered drug in the urine, followed by modest
components of metabolites N-oxide (about 4%-6%), s-oxide
(about 1%-2%), dimethyl-ranitidine (about 1%-2%) and furic
acid analognes (1%-2%) [10]. As a result of its importance as
a filtration organ, this medication has an effect on the kidney.
Oxidative deamination forms the furic acid analogues. It’s likely
that the formation of this metabolite, as proposed by oxidative
deamination, will also result in the release of dimethylamine
(DMA), which might then have been used to make NDMA when
exposed to nitrite. However, in this publication it has been describing
human metabolism data [11].
According to the report of Galaxosmith, (2012) in individuals
with renal impairment (creatinine clearance less than 50 ml/min), ranitidine accumulates in the bloodstream, leading in
high plasma concentrations. In such patients, a daily oral zantac
dose of 150 mg is indicated, with a zantac injectable dose of 25
mg. In patients with renal impairment, however, ranitidine is
eliminated through the kidney, resulting in higher drug plasma
levels. In renal impairment, the dose should be increase as described
above under dosage and administration.
Conclusion
The ranitidine can cause hemorrhage and focal inflammation
and desquamation in the central vein and an expansion in the
sinusoid of the liver, also can cause hemorrhage and necrosis
and the presence of cast in the tubules of kidneys, these increase
with the increased doses.
Declarations
Ethics Approval
The laboratory animals were placed in special cages and left
for a week to acclimatize, a special diet and sterile water were
used, and the hours of light, darkness and the appropriate temperature
were taken into account in the animal house of the
college of Veterinary/University of Tikrit, according to national
and international guidelines for the care and use of laboratory
animals, and approved by the animal care and use committee,
collage of Veterinary/University of Tikrit, the command with
the number 3372/14 in 13/1/2021.
Competing Interest
The author has no financial interest to disclose.
Funding
No funding
Availability of Data Materials
https://docs.google.com/file/d/1vnLD3b2qXuTVtWg73H_
X0JOVzr3E2Ln5/edit?usp=docslist_api&filetype=msword
References
- Zantac side effects. Florida: Drugwatch.
- Colombo JA, Wagner JM (2020) Medicine and media: The ranitidine debate. Clin Transl Cci. 13(4):649-651.
[Crossref] [Google Scholar]
- Fiona JK, Searle AD, Urquhart MW (2020) Ranitidine-Investigations into the root cause for the presence of N-Nitroso-N, N-dimethylamine in ranitidine hydrochloride drug substances and associated drug products. J Am Chem Soc. 24(12):2915-2926.
[Crossref] [Google Scholar]
- FDA updates and press announcements on NDMA in zantac (ranitidine). FDA.
[Google Scholar]
- Mahase E (2019) FDA recalls ranitidine medicines over potential cancer causing impurity. BMJ. 367:l5832.
[Crossref] [Google Scholar]
- U.S. Food & Drug Administration (2019) Statment alerting patients and health care professionals of NDMA found in samples of ranitidine. FDA.
[Google Scholar]
- Suvarna SK, Layton C, Bancroft JD (2019) Bancofts theory and practice of histological techniques. 8th Edition.
[Google Scholar]
- Faried AE, El-sayed KA, Mostafa AA (2005) Biochemical and histological studies on H2-receptor antagonist ranitidine-induced hepatotoxicity in rats. Indian J Exp Bio. 43(9):782-785.
[Google Scholar]
- Bell JA, Dallas FA, Jenner WN, Martin LE (1980) The metabolism of ranitidine in animals and man. Biochem Soc Trans. 8(1):93.
[Crossref] [Google Scholar]
- Martin LE, Oxford J, Tanner RJ (1982) Use of high-performance liquid chromatography mass spectrometry for the study of the metabolism of ranitidine in man. J Chromatog. 251(2):215-224.
[Crossref] [Google Scholar]
- Martin LE, Oxford J, Tanner RJ (1981) The use of on-line high-performance liquid chromatography-mass spectrometry for the identification of ranitidine and its metabolites in urine. Xenobiotica. 11(12):831-40.
[Crossref] [Google Scholar]
Citation: Rashid MS (2023) Histopathological Effect of Ranitidine (Zantac) on Liver and Kidneys in Albino Mice. J Clin Gastroenterol
Hepatol. 7:1.
Copyright: © 2023 Rashid MS. This is an open-access article distributed under the terms of the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source
are credited.




