Research Article - (2025) Volume 11, Issue 5
Experimentation of the IFAST Technique for DNA Extraction Using Two Different Methods
Saad Butt*,
Muhammad Faisal,
Hammad Ali Sajid and
Muhammad Imran Shabbir
Department of Biotechnology, International Islamic University, Islamabad, 44000, Pakistan
*Correspondence:
Saad Butt, Department of Biotechnology, International Islamic University, Islamabad, 44000,
Pakistan,
Email:
Received: 19-Mar-2024, Manuscript No. IPBMBJ-24-19254;
Editor assigned: 21-Mar-2024, Pre QC No. IPBMBJ-24-19254 (PQ) ;
Reviewed: 05-Apr-2024, QC No. IPBMBJ-24-19254;
Revised: 21-Feb-2025, Manuscript No. IPBMBJ-24-19254 (R) ;
Published:
28-Feb-2025
Abstract
Purpose: Immiscible Filtration Assisted by Surface Tension (IFAST) is a microfluidic technique that uses the principle of surface tension for the extraction of analyses such as nucleic acids, proteins, and metabolites. This immiscible phase filtration technique was tested via two different cost-effective methods.
Methods: Two cost-effective methods for applying the IFAST technique were developed and the procedures were compared for effectiveness. These methods involved the use of plastic canvas and Eppendorf tubes for device fabrication and DNA extraction.
Results: In the first method, several challenges are faced, which are discussed in this research, where plastic canvas is used instead of expensive materials such as Polydimethylsiloxane (PDMS). On the other hand, the air-jump method is better for DNA extraction because of the new approach of using Eppendorf tubes for IFAST.
Conclusion: Applying the IFAST technique using the air-jump method is a relatively more robust, economical and effective method than the use of a plastic canvas device.
Keywords
Microfluidics; DNA extraction; IFAST; Paramagnetic particles; Immiscible filtration; Air-jump
Introduction
Immiscible filtration assisted by surface tension (IFAST) is an immiscible phase filtration system designed for the extraction of nucleic acids, proteins, and cells [1]. This technique uses the principle of surface tension for adjusting the liquids in wells. There is an immiscible barrier with low surface tension (in the middle phase), that separates the sample and the elution buffer. In addition, Paramagnetic Particles (PMPs) are used for capturing the specific analyte in the sample. In this process, nucleic acid binds to paramagnetic particles and then passes through the oil phase for the filtration. After filtration through the oil phase, the purified nucleic acid is moved towards the elution buffer. In this way, three phases can be set in a series with the help of surface tension, to extract the specific analyte.
This is a microfluidic technique for the extraction of specific analytes from biological samples. Microfluidics is a system that is used for handling and analyzing fluids at the micrometer level [2]. A microfluidic platform offers a collection of fluidic unit operations that can be combined with ease inside a welldefined fabrication method. The integration, automation, parallelization, and shrinking of biochemical processes are made possible by a microfluidic platform, which offers a universal and uniform method [3]. It is beneficial for laboratories because it requires less volume of a liquid, a small device size, and lower energy consumption. The channels are designed in microfluidics devices for the flow of liquid in a specific direction. It is used for Lab-on-a-Chip (LOC) technology in which several laboratory functions are integrated on a single chip.
Instead of using expensive materials, such as Polydimethylsiloxane (PDMS), for fabricating the IFAST device, an easily available and inexpensive material i.e., plastic canvas, is used. On a plastic canvas, three wells were designed in series for lysed solution (with sample and paramagnetic particles), oil, and elution buffer [4]. The channels were made between the wells for the transfer of paramagnetic particles from one well to another. The wells of the plastic canvas were sealed from both sides with the ‘Microseal B adhesive sealer from bio-rad’. This method does not require any external equipment other than syringes and magnet, unlike other microfluidic methods, which require multiple electronic and hydraulic connections, making it difficult to perform outside the setting laboratory setting [5].
The air-jump method is a simple method for the extraction of PMP-bound analytes by applying the magnetic force from above and by jumping through the air, the PMP-bound analytes are purified and transferred to another tube [6]. For this method, two Eppendorf tubes were used for performing the IFAST technique. In the first tube, the lysed solution with paramagnetic particles, and oil were added, while the second Eppendorf tube was used for the elution buffer. Paramagnetic particles were filtered and isolated from one tube using a magnet, and then inserted into the elution buffer in the second tube. Finally, the eluted analyte is extracted from the elution phase.
The extraction of specific analytes is an essential process in various fields such as biological research, forensics, diagnostics, and genetics, etc. In this research, the IFAST technique was applied in two different ways to test the effectiveness of the methods. Furthermore, the purpose of proposing these methods for the extraction of nucleic acid, i.e., DNA, in this research is to provide ease to researchers and to introduce a cost-effective method. The two different methodologies were compared to determine the best method for DNA extraction. Moreover, the problems of performing these methods are also discussed in this research (Figure 1).

Figure 1: IFAST model designed on plastic canvas.
Materials and Methods
Materials and reagents: This research project focuses on the development and optimization of a robust DNA extraction protocol for molecular biology applications. The study employed a range of materials and reagents, including plastic canvas, microseal B adhesive sealer from bio-rad (PCR Tape), insulin syringes, olive oil, a plastic cutter, Eppendorf tubes, pipettes, a neodymium magnet, a water bath, a nanodrop spectrophotometer, an NA Universal Auto Extraction Kit and saliva sample. The utilization of these materials is crucial for ensuring the efficiency, precision, and reliability of the DNA extraction process.
Device Design, Fabrication, and Preparation
Plastic canvas design and fabrication: We developed the present device utilizing a grid structure constructed from Polyvinyl Chloride (PVC) plastic. The fabrication process involved precision cutting of the plastic using a specialized cutter to create a series of three square-shaped wells arranged linearly on the grid. Channels were strategically introduced between the wells to facilitate the seamless movement of Paramagnetic Particles (PMPs) from one well to another. Each well was designed to have a volumetric capacity of approximately 120 microliters. Subsequently, the wells were sealed using ‘micro-seal B adhesive sealer from Bio-Rad’ on both sides. This tape was chosen for its robust adhesive properties, ensuring effective containment of various reagents such as lysis solution, elution buffer, and even immiscible oil within the individual wells. We added lysis solution containing PMPs to the 1st well and elution buffer to the 3rd well, and oil was added in between these two wells to create an immiscible barrier. This is how we design device on canvas to perform Immiscible Filtration Assisted by Surface Tension (IFAST). This methodical design not only provides a controlled microenvironment for the particles but also enhances the overall efficiency and functionality of the device for specific experimental applications (Figure 2).
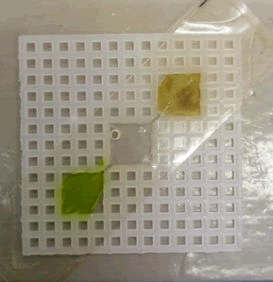
Figure 2: Plastic canvas device.
Air jump method design and fabrication: Previously, elution wells set up as a strip or plate, a magnet holder, a regular 96- well plate (Falcon), and a base with alignment posts make up the Air-Jump apparatus. Stereolithography (SLA) was used to 3D print the base and magnet holder using accura 25 plastic, which is similar to polypropylene (Midwest Prototyping). Plates with 96 elution wells are 3D printed in accura 25, while strips with 8 elution wells are injection-compression molded in polypropylene.
In the pursuit of optimizing simplicity and cost-effectiveness, we executed the air-jump method employed by IFAST within the confines of a straightforward Eppendorf tube. The method involves meticulous preparation and execution, utilizing a purpose-designed apparatus to facilitate the procedure. The requisite concentration of lysis solution, augmented with paramagnetic particles, was introduced into the Eppendorf tube. Above this solution, an immiscible fluid, typically in the form of oil, is carefully layered. The elution buffer was added to a separate tube. The operational intricacies of the air-jump method are seamlessly conducted within this specialized device. This innovative approach enhances the efficiency and efficacy of the methodology, ensuring controlled and reproducible execution. The systematic incorporation of the Lysis solution with PMPs and the strategic implementation of the immiscible fluid create a conductive environment for the Air Jump method to be executed with precision and reliability. This nuanced protocol not only streamlines the procedure but also contributes to the reproducibility and scalability of the IFAST technique (Figure 3).

Figure 3: Air jump kit: (a) Lysed solution with PMPs+oil, (b) Elution buffer
Loading on Devices
Loading on canvas: In the course of loading the experimental platform, an insulin syringe was employed to meticulously introduce solutions into individual wells, each of which was carefully perforated on PCR tape to preclude the formation of air bubbles during the loading process. This precaution was taken to ensure the integrity of subsequent solution placement within the wells. Employing the Immiscible Filtration Assisted by Surface Tension (IFAST) methodology, particular attention was given to the loading sequence due to the reliance on surface tension for the precise positioning of liquids within the device. Consequently, the oil component, characterized by its exceptionally low surface tension, was introduced as the final step in the loading sequence, serving to establish an immiscible barrier. The loading protocol commenced with the introduction of a lysis+sample (lysed solution) containing Paramagnetic Particles (PMPs) into the first well known as the input well, followed by the subsequent loading of the third well known as the output well with elution buffer. In conclusion, the middle well was filled with olive oil to strategically create an effective immiscible barrier. Notably, each well received a uniform volume of 120 μL of the corresponding solution, ensuring consistent well-filling across the array. Prior to the commencement of the operational phase, a visual inspection of the loaded arrays was conducted to confirm the accurate placement of all reagents. This visual verification step served as a crucial quality control measure, enhancing the reliability of subsequent experimental procedures.
During preliminary loading demonstrations, water infused with food coloring was substituted for the lysis and elution solutions. This deliberate substitution facilitated an assessment of the reliability and retention characteristics of the loading process, serving as an invaluable tool for refining and troubleshooting the experimental protocol.
Loading in air jump method: The Eppendorf tube was loaded using a meticulous approach involving both a pipette and an insulin syringe, in accordance with the air-jump method. The loading sequence involved the precise addition of reagents to facilitate optimal separation and extraction of DNA. Commencing the procedure, the lysed solution, consisting of a combination of lysis reagent and the biological sample of interest, was carefully dispensed into the Eppendorf tube using a pipette. This initial step ensured the introduction of the biological material into the experimental system. Subsequently, an immiscible barrier was created within the Eppendorf tube by adding olive oil above the lysed solution, facilitated by the controlled use of an insulin syringe. The immiscible barrier, a critical component of the air-jump method, served to isolate the lysed solution and prevent intermixing with subsequent reagents, contributing to the precision and efficacy of the DNA extraction process.
The concentration of the lysed solution within the Eppendorf tube was maintained within the range of 150-200 μL, ensuring an optimal volume for subsequent steps. This concentration was precisely mirrored when adding olive oil above the lysed solution, ensuring consistency in experimental conditions. In a separate Eppendorf tube, 100-120 μL of elution buffer was added using a pipette. Elution buffer, a critical component in DNA extraction, serves to release and isolate the extracted DNA. The measured and controlled addition of elution buffer ensures the appropriate conditions for efficient DNA elution.
This loading procedure, characterized by meticulous attention to detail and adherence to specific concentrations, forms the foundation for subsequent stages of the air-jump method. Such precise techniques are essential in molecular biology applications, ensuring the reliability, reproducibility, and accuracy of experimental outcomes.
Operation of Devices
The protocol of the ‘NA universal auto extraction kit’ was followed because DNA extraction was carried out in accordance with the kit. This extraction kit contained all of the reagents needed for the IFAST operation, including the lysis solution containing PMPs and the elution buffer.
Sample lysis: Saliva samples underwent an initial lysis step in accordance with the ‘NA universal auto extraction kit’ protocol within an Eppendorf tube before loading onto the IFAST device. The Eppendorf tube, harboring both the lysis solution (ranging from 80-120 μL) and the saliva sample (ranging from 45-65 μL), was subjected to a controlled thermal treatment in a water bath set at 85â?? for a duration of 8 minutes. To optimize sample lysis, the tube was manually agitated at oneminute intervals throughout the incubation period. This meticulously regulated process aimed to enhance the efficiency of sample lysis and thereby facilitate subsequent molecular analyses (Figure 4).

Figure 4: Lysis of sample in water bath at 85°C.
Plastic canvas device operation: Once the plastic canvas device was filled with the requisite solutions, it underwent operational procedures facilitated by an external neodymium magnet. The magnet, affixed to a magnet holder, was strategically positioned at the base of the initial well containing the lysed solution. According to the kit, the magnet remained stationary beneath this input well for duration of 60 seconds. This temporal interval was designated to facilitate the attraction of PMPs, which were bound to DNA, toward the magnet. Following this phase, the magnet was methodically and deliberately moved in a slow and steady manner toward the middle well, which housed olive oil, through a designated channel. This well functioned as an immiscible phase and played a pivotal role in the filtration of DNA bound to PMPs, while concurrently eliminating impurities. The magnet was held in place beneath the oil well for a minimum of 3 minutes, ensuring effective separation and purification.
Subsequently, the magnet was gradually moved toward the output and final well, which contained elution buffer, through a designated channel. The magnet remained well positioned at the base of the elution buffer well for a period of 90 seconds. This strategic maneuver resulted in the elution of DNA in the third well, thereby affecting the detachment of DNA from the PMPs (Figure 5).
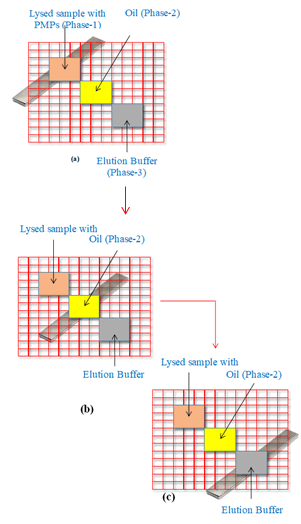
Figure 5: (a) Magnet was kept beneath phase-1 for 60 seconds, (b) Magnet was kept beneath phase-2 for 3 minutes, (c) Magnet was kept beneath phase-3 for 90 seconds.
Air jump method operation: Following the loading of the lysed solution and olive oil into the Eppendorf tube, the Air Jump method, assisted by the Immiscible Filtration Assisted by Surface Tension (IFAST) technique, was performed in accordance with the ‘NA universal auto extraction kit’ protocols. A neodymium magnet affixed to a magnet holder and positioned externally at the tube's base was applied for 60 seconds. This action facilitated the attraction of PMPs, attached to DNA within the lysed solution, toward the magnet. Subsequently, the magnet was slowly moved from its external position toward the oil phase above the lysed solution. The magnet was placed beside the oil within the tube for a minimum of 3 minutes, exploiting the oil's immiscible nature to effectively filter the DNA attached to the PMPs and eliminate impurities. Following this filtration step, a plastic-tube-covered neodymium magnet was introduced into the Eppendorf tube from above. This entry prompted the PMP-bound DNA within the oil phase to jump from the oil and adhere to the magnet. The magnet, now carrying the PMPs, was then transferred to another Eppendorf tube containing elution buffer. The release of PMP-bound DNA into the elution buffer was achieved by inserting the magnet, which was covered with a plastic tube and bearing attached PMPs, into the elution buffer tube from above. By pulling the magnet above, PMP-bound DNA adhering to the plastic tube was released into the elution buffer. Subsequently, the elution buffer tube was placed in a water bath at 85°C for 2 minutes, with manual shaking occurring every 30 seconds to facilitate proper elution. This process resulted in the detachment of DNA from the PMPs.
To conclude, the magnet was positioned at the bottom of the elution buffer tube for 90 seconds, and the buffer containing the eluted DNA was collected from above. This comprehensive procedure demonstrates a meticulous and controlled method for the selective extraction and elution of DNA using the airjump technique in conjunction with the IFAST approach (Figure 6).
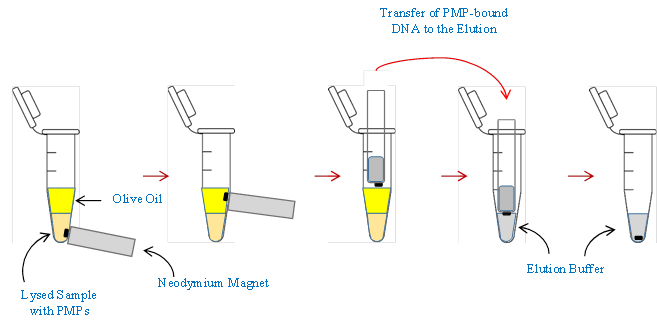
Figure 6: IFAST technique using air jump method.
Analysis
The analysis of extracted DNA in elution buffer involves utilizing a nanodrop spectrophotometer, a widely employed instrument in molecular biology laboratories for quantifying and evaluating nucleic acid samples, particularly DNA. Spectrophotometry is employed to measure light absorption at specific wavelengths by substances in solution, with DNA exhibiting absorption at approximately 260 nanometers (nm).
The procedure commences with the initiation of the software connected to the nanodrop spectrophotometer. We selected the desired application, focusing on nucleic acids. Subsequently, the hinged arm, situated horizontally on the instrument, is gently raised, revealing a small dark spot denoting the pedestal for sample placement. Thorough cleaning and buffing of the pedestal with a lint-free wipe preceded the pipetting of 1-2 μL of the blanking solution. The arm was then gently lowered, and "Measure blank" was selected. Following the measurement of the blank, the arm was lifted again, and the pedestal was cleaned with a lint-free wipe. This sequence was repeated, with the blanking solution replaced by the DNA sample. The introduction of a minute volume (typically 1-2 microliters) of the prepared DNA sample occurs on the micro-volume pedestal of the nanodrop instrument.
The nanodrop spectrophotometer employs a UV-Vis spectrophotometer to emit a UV light beam through the DNA sample. The absorbance of light at 260 nm was measured to determine the concentration of nucleic acids, while absorbance at 280 nm is assessed to gauge protein contamination, aiding in purity estimation. The ratio of the absorbance at 260 nm to that at 280 nm serves as a metric for evaluating DNA purity, with a ratio of approximately 1.8 indicating relatively pure DNA. Information acquired from the NanoDrop is instrumental in assessing DNA sample quality, with high-quality samples typically exhibiting a high 260/280 ratio, signifying minimal contamination.
Results
Device Fabrication and Loading using Plastic Canvas
Firstly, a normal plastic tape was tried to cover the upper and lower side of the wells. Water was injected in the first and third phases, and the middle phase was filled with cooking oil. Below are the images of the canvas. The tape was not adhesive. As a result, water and oil moved away from their respective wells. Therefore, the tape needs to be replaced with another adhesive material (Figure 7).
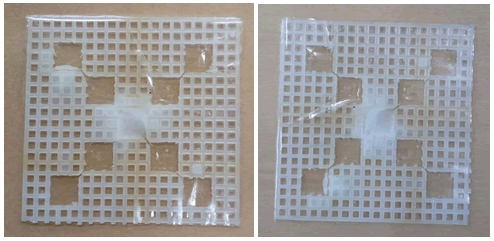
Figure 7: Water and oil leaked from the wells of the plastic canvas.
When the book binding tape and double-sided transparent tape were used to cover the lower and upper sides of the canvas, respectively. Both tapes worked well for adhesion. Fluid remained in its respective wells for a small period of time. After a few seconds, water and oil leakage started (Figure 8).

Figure 8: Size of canvas reduced to one passage. Fluid remained for a small period of time.
When one canvas covered with double-sided transparent tape was compared with the other canvas covered with binding tape on the lower side and double-sided transparent tape on the upper side, it was observed that the first canvas worked better than the second one because the fluid remained in the wells. However, while taking and injecting the oil, air comes with it and makes its way out from the well that causes problem (Figure 9).
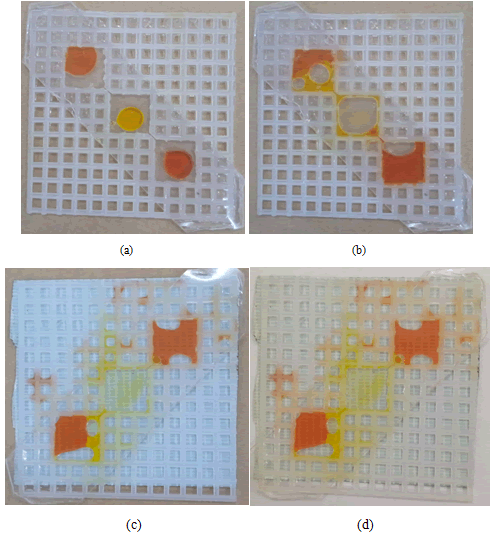
Figure 9: (a,b) Double-sided transparent tape on both sides. (c,d) Binding tape on the lower side and double-sided transparent tape on the upper side.
The first process was repeated again for more clarity. Now, the only issue is with oil because it starts leaking after 1 minute of injecting into the well. Below are the images of the canvas (Figure 10).
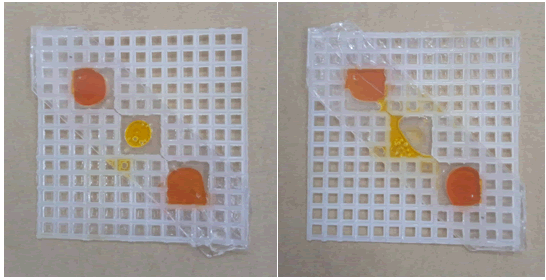
Figure 10: Oil leakage from its well.
Now, when the following two cases are compared, i.e.:
• Double-sided transparent tape between two canvas of the same size. The upper and lower side were also covered (Figure 11).

Figure 11: (a) Upper side view. (b) Lower side view. Oil leaked from the well.
• Single canvas with double sided transparent tape on both sides (Figure 12).

Figure 12: Oil leakage from the tape.
Water remains in its respective wells, but oil reduces the stickiness of the tape and leaks from it. Double-sided transparent tape is good for water but cannot retain oil.
Silicone sealant is also used for blocking the boxes of canvas near the wells. However, the double-sided transparent tape was not able to attach to it because silicone sealants typically have low surface energy. Adhesion is generally better on surfaces with higher surface energy. Double-sided tapes often adhere better to high-energy surfaces such as glass, metal, or certain plastics. Silicone has a low surface energy, which can make it challenging for adhesives to bond effectively.
Furthermore, a glue gun is used to block the boxes or spaces near the wells. As a result, double-sided transparent tape was attached to the surface, which was layered by a glue gun. Water remained in its respective wells but the oil affected the tape. Oil reduces the stickiness of the tape, loses the tape and eventually leaks from the well (Figure 13 and 14).

Figure 13: (a) Double-sided transparent tape on glue. (b) Water filled in the wells.
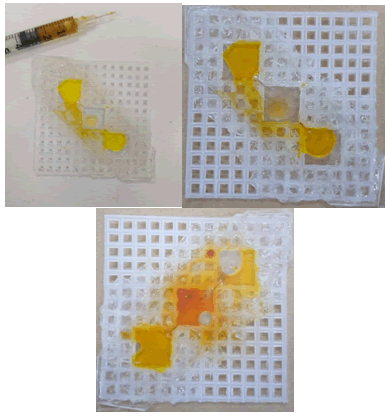
Figure 14: Oil leaked from its well.
Moreover, oil leaks from the upper side by solubilizing the tape, thus reducing the stickiness of the tape when the plastic cover of the mobile protector is attached to the lower side of the canvas with a glue gun, and double-sided transparent tape is used to cover the upper side (Figure 15).
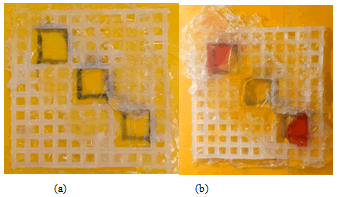
Figure 15: (a) Plastic cover on the lower side of canvas and double-sided transparent tape on the upper side. (b) Oil leakage from the well.
The oil was then replaced with n-hexane. N-hexane (C6H14) is a colorless liquid with a characteristic odor. It has a boiling point of approximately 68.7 degrees Celsius (155.7 degrees Fahrenheit) and is relatively nonpolar. In this case, n-hexane was dissolved with the tape, and only the color of the permanent marker ink used to dye the n-hexane remained on the surface of the tape (Figure 16).

Figure 16: (a) n-hexane loaded into the well of Phase-2. (b) nhexane colored with permanent marker ink.
After the failures discussed above, a new way of fabricating the device was attempted. Firstly, the double-sided transparent tape was attached to the lower side of the canvas. Then, a layer of transparent colorless nail polish was added to the surface of double-sided transparent tape (in oil well only). Secondly, double-sided transparent tape (with a layer of colorless nail polish) was attached immediately after applying nail polish to its surface to the upper side of the canvas (oil-only phase). In addition, nail polish is also applied on the corners of water wells. As a result, this method worked well but was not very effective. Oil remained in its well and water leaked from one well because of the canvas. During the second attempt, oil leaked from the well and water remained in the wells. Therefore, this way of fabricating the device is not very effective (Figure 17).

Figure 17: Nail Polish is applied in the oil phase and on the corners of water wells.
Finally, when the ‘Microseal B Adhesive Sealer from Bio-Rad’ is used to seal the upper and lower sides of the wells in the canvas, then both water and oil remained in their wells. However, one well of water was not designed correctly. Therefore, water did not remain within its well after 15 minutes and moved to the well of oil that had air bubble in it (Figures 18 and 19).

Figure 18: (a) Water injected into its wells. (b) Oil injected into its well.

Figure 19: (a-d): Stage of water movement from phase-1 to phase-2.
This same process was repeated again. As a result, oil and water remained in their wells. Thus, the IFAST model on a plastic canvas is fabricated successfully.
Results of device operation on plastic canvas: The same process was used for running paramagnetic particles without sample. The PMPs successfully passed through the oil phase and entered the third phase. However, while passing the PMPs, the channel was not large enough to allow the PMPs to pass at one time. Therefore, the channel width needs to be expanded. In addition, the formation of air bubbles due to oil is also a problem faced during the movement of Paramagnetic Particles (PMPs) by magnets from phase-1 to phase-2 (Figure 20).

Figure 20: Testing the movement of paramagnetic particles.
When the sample (saliva) was lysed in water bath at 85°C and after lysis, it was loaded into the well of canvas. The magnet was kept under the 1st well for 60 seconds and then moved toward the oil phase. In the oil phase, canvas was subjected to PCR at 85°Cfor 2 minutes. As a result, all the liquid, i.e., elution buffer, oil, and the liquid in phase-1, leaked and evaporated. Providing temperature to the solution inside the canvas is the main hindrance in performing the IFAST technique in a plastic canvas because the temperature reduces the stickiness of the tape, and eventually, the liquid leaks from the wells. Only the paramagnetic particles are left in the well.
Results of operation using the air-jump method: At first, DNA cannot be filtered effectively because of the tape used for wrapping the magnet, the size of the tubes, and some handling errors. The results obtained with a nanodrop spectrophotometer are shown in Table 1.
| First report |
Second report |
Third report |
| Conc.: 394.261 ng/µl |
Conc.: 311.131 ng/µl |
Conc.: 283.061 ng/µl |
| A260: 7.885 |
A260: 6.222 |
A260: 5.661 |
| A280: 7.594 |
A280: 5.656 |
A280: 5.223 |
| A260/A280: 1.04 |
A260/A280: 1.1 |
A260/A280: 1.08 |
Table 1: Nucleic acid detection report.
The same process of Air Jump method was repeated. TBE buffer was used as an elution buffer now. However, TBE buffer is not good for the elution of DNA because of the presence of borate, which is used for gel electrophoresis. The results are shown in Table 2.
| First report |
Second report |
Third report |
| Conc.: 138.61 ng/µl |
Conc.: 128.515 ng/µl |
Conc.: 76.616 ng/µl |
| A260: 2.772 |
A260: 2.57 |
A260: 1.534 |
| A280: 2.853 |
A280: 2.642 |
A280: 1.454 |
| A260/A280: 0.97 |
A260/A280: 0.97 |
A260/A280: 1.05 |
Table 2: Nucleic acid detection report.
The same process was repeated with olive oil and TE buffer for elution. A small Neodymium magnet was used to cover the tube so that could easily enter the Eppendorf tube and attract the paramagnetic particles to it. The results of nucleic acid detection using a Nanodrop spectrophotometer are shown in Table 3 and Figure 21.
|
First report
|
Second report
|
Third report
|
|
Conc.: 57.04 ng/µl
|
Conc.: 62.181 ng/µl
|
Conc.: 65.61 ng/µl
|
|
A260: 1.14
|
A260: 1.243
|
A260: 1.312
|
|
A280: 1.128
|
A280: 1.245
|
A280: 1.302
|
|
A260/A280: 1.01
|
A260/A280: 1.0
|
A260/A280: 1.01
|
Table 3: Nucleic acid detection report.
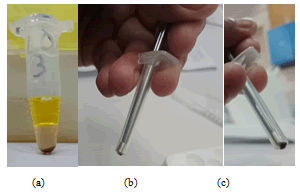
Figure 21: (a) Lysed sample with PMPs and the layer of olive oil above it. (b, c) Neodymium magnet covered with a thin tube.
The A260/A280 ratio is a measurement obtained from a spectrophotometer, such as a Nanodrop spectrophotometer, and it is used to assess the purity of nucleic acid samples, such as DNA and RNA. The ratio was calculated by dividing the absorbance at 260 nm (A260) by the absorbance at 280 nm (A280). A260 is the absorbance of light at a wavelength of 260 nanometers. At this wavelength, nucleic acids, particularly DNA and RNA, strongly absorb light. Therefore, the A260 value is used to quantify the amount of nucleic acids in a sample. A280 is the absorbance of light at a wavelength of 280 nanometers. Proteins absorb light strongly at this wavelength. Therefore, the A280 value is used to estimate the amount of protein contamination in a nucleic acid sample. The A260/A280 ratio is an important indicator of nucleic acid purity. A pure nucleic acid sample, without significant protein contamination, will have a higher A260/A280 ratio. For DNA, a typical pure sample might have a ratio of approximately 1.8, while for RNA, the ratio is often higher, approximately 2.0. A low A260/A280 ratio may suggest protein contamination in the nucleic acid sample, while a high ratio may indicate contamination with other substances or that the sample is not pure nucleic acid. Monitoring this ratio is crucial in molecular biology and biochemistry, especially when working with sensitive techniques such as PCR or RNA analysis.
Discussion
In this study, DNA extraction via the IFAST method was accomplished utilizing a plastic canvas device and the air-jump technique. The plastic canvas device, constructed from a costeffective PVC plastic grid, features three wells created with a cutter accompanied by channels. These wells were effectively sealed using a ‘Microseal B Adhesive Sealer from Bio-Rad’ on both sides, rendering the device easily fabricable and economically advantageous. In contrast, IFAST devices fabricated from Polydimethylsiloxane (PDMS) proved to be more expensive than the device fabricated using plastic canvas.
To ensure simplicity and cost-effectiveness during the air jump method, the procedure was adeptly executed within a standard Eppendorf tube. The tube accommodated the requisite concentration of Lysis solution with PMPs, topped with an immiscible fluid (oil). This uncomplicated setup facilitated the smooth operation of the air-jump method within the Eppendorf tube. Notably, this approach contrasts with the conventional airJump apparatus, which typically comprises a base with alignment posts, a 96-well plate, elution wells configured as strips or plates, and a magnet holder. The conventional apparatus, constructed from Accura 25 plastic via stereolithography and polypropylene through injection-compression molding, presented a higher cost than the more economical Eppendorf-based alternative.
In plastic canvas device, some difficulties and challenges are faced during the process of applying the IFAST technique for DNA extraction. It is a closed microfluidic system, in which there is a less chance of contamination and requires high manipulation skills. During the loading of oil in its well, the air bubbles are formed which makes it difficult for the PMPbound DNA to pass from phase-1 to phase-2. When the PMPbound DNA is suspended in the oil phase (phase-2), it needs the temperature of 85â?? as mentioned in the protocol of the kit. When the temperature of 85â?? was applied to the canvas in phase-2 of the process in PCR (as mentioned above in the Results section), the adhesive properties of the tape were reduced at that temperature and eventually all the liquid leaked from the wells of the canvas. Even if the temperature is not given in the oil phase and the PMP-bound DNA is just transferred to the elution phase, the same challenge of the tape would be faced at that time because the elution phase (phase-3) also requires a temperature of 85â?? which reduces the adhesive properties of the tape. Moreover, the PMPbound DNA is not possible to be taken out using insulin syringe from the elution phase for providing the temperature.
In the air-jump method, Eppendorf tubes are used for the performing the IFAST technique for DNA extraction. It is an open system, in which there is a more chance of contamination than the close system. This method does not require high manipulation skills and can be easily performed. In this method, there is no formation of air bubbles, unlike in a plastic canvas device. Providing the temperature is not an issue in this method because the Eppendorf tubes can easily withstand the temperature from -86â?? to 100â??. In this way, an inexpensive way of performing the IFAST technique was developed (Table 4).
| Characteristics |
IFAST using plastic canvas |
IFAST using Air-Jump |
| Cost |
Inexpensive |
Inexpensive |
| Working time |
Takes more time |
Takes less time |
| Formation of air bubbles |
Yes |
No |
| Status of system |
Closed system |
Open system |
| Manipulation skill |
Difficult |
Easy |
| Applying temperature |
Difficult |
Easy |
| Risk of contamination |
Very less |
Relatively high |
| Transfer of PMP-bound DNA from one phase to another |
Difficult due to air bubbles |
Easy |
| Providing the temperature in oil phase |
Difficult |
Difficult |
| Results |
No |
Yes |
| Effectiveness |
Not effective |
Effective |
Table 4: Comparison of the IFAST technique using plastic canvas with the IFAST technique using the air-jump method.
The air-jump kit employed in the study demonstrated superior effectiveness and reliability compared to the plastic canvas device. The plastic canvas device posed challenges in maintaining a consistent temperature, particularly when the PMPs were immersed in oil or during the elution process. Temperature fluctuations were found to compromise the adhesive properties of the PCR tape, leading to solution leakage. In contrast, the air-jump method facilitated seamless DNA extraction, establishing its preference over the plastic canvas device. This preference is attributed to the ability of the air-jump method to ensure temperature stability throughout the procedure, enhancing the overall reliability and efficiency of DNA extraction.
This research was conducted to introduce cost-effective and easily manipulated methods for performing the IFAST technique for DNA extraction. The limitations of these methods includes the formation of air bubbles in plastic canvas, high manipulation skills for plastic canvas device, and maintaining temperatures, as discussed above. Although the results of the air-jump method are not as expected, despite these challenges, the air-jump method is found to be the more effective, easily manipulated and inexpensive method as compared to the plastic canvas device. This method can be used in various fields including forensics, biological research, paternity testing, and genetics, etc. In addition, this research paves the way for future researches and can be used for developing the ways in future that can overcome the challenges faced during the process. The effectiveness of these methods would be enhanced if an inexpensive and easily manipulated method would be introduced.
Conclusion
In conclusion, the Immiscible Filtration Assisted by Surface Tension (IFAST) technique is applied in two different ways to introduce a cost-effective and easily performed method. This technique has been used previously for the extraction of analytes, e.g., nucleic acids, proteins, and metabolites, but the fabrication of the device in that method was expensive. In this research, we experimented to develop an inexpensive, effective, and easily used method. The air-jump method is a relatively effective method for DNA extraction in comparison with the device fabricated using plastic canvas because of its simplicity and low-time consumption. The effectiveness of these methods could be enhanced in the future, which can lead to more robust and successful methods for DNA extraction.
Statements and Declarations
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Author Contributions
All authors contributed to the study conception and design. Dr. Muhammad Imran Shabbir was a supervisor. Saad Butt, Muhammad Faisal, and Hammad Ali Sajid performed data collection, material preparation, and analysis. Saad Butt and Hammad Ali Sajid wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- Beebe DJ, Mensing GA, Walker GM (2022) Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 4(1):261-286.
[Crossref] [Google Scholar] [PubMed]
- Berry SM, Pezzi HM, LaVanway AJ, Guckenberger DJ, Anderson MA, et al. (2016) AirJump: Using interfaces to instantly perform simultaneous extractions. ACS Appl Mater Interfaces. 8(24):15040-15045.
[Crossref] [Google Scholar] [PubMed]
- Berry SM, Regehr KJ, Casavant BP, Beebe DJ (2013) Automated operation of immiscible filtration assisted by surface tension (IFAST) arrays for streamlined analyte isolation. J Lab Autom. 18(3):206-11.
[Crossref] [Google Scholar] [PubMed]
- Eppendorf (2015) Eppendorf Tubes. Product Manual. 1–21.
- Mark D, Haeberle S, Roth G, Von Stetten F, Zengerle R (2010) Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev. 305-376.
[Crossref] [Google Scholar] [PubMed]
- Strotman LN, Lin G, Berry SM, Johnson EA, Beebe DJ (2012) Facile and rapid DNA extraction and purification from food matrices using IFAST (immiscible filtration assisted by surface tension). Analyst. 137(17):4023-4028.
[Crossref] [Google Scholar] [PubMed]
Citation: Butt S, Faisal M, Sajid HA, Shabbir MI (2025) Experimentation of the IFAST Technique for DNA Extraction Using Two Different Methods. Br J Res. 11:47.
Copyright: �© 2025 Butt S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






















