- (2010) Volume 11, Issue 4
Susan Alsamarai1, Steven K Libutti2, Muhammad Wasif Saif1
1Yale Cancer Center, Yale University School of Medicine. New Haven, CT, USA.
2Montefiore-Einstein Center for Cancer Care, Montefiore Medical Center, Albert Einstein College of Medicine. Bronx, NY, USA
Pancreatic neuroendocrine tumors display a range of clinical presentations and outcomes. Surgical resection remains the only potentially curative approach for primary tumors, and is also associated with a survival benefit for hepatic metastases as well. Data presented at the American Society of Clinical Oncology (ASCO) Annual Meeting this year suggest that targeted agents may also play a role in advanced disease. Sunitinib, which targets VEGF-1, 2 and 3 and PDGF seems to be a well tolerated treatment for advanced tumors. The mTOR inhibitor everolimus when combined with the VEGF inhibitor bevacizumab, resulted in measurable responses. The combination of bevacizumab and cytotoxic chemotherapy also shows potential.
bevacizumab; mTOR protein; Neuroendocrine Tumors; sunitinib
ASCO: American Society of Clinical Oncology; MGMT: O-6-methylguanine-DNA methyltransferase; SEER: Surveillance, Epidemiology and End Results
Pancreatic neuroendocrine tumors (PNETs) are relatively rare and generally felt to follow an indolent course. However, when poorly differentiated or metastatic, these tumors can also behave in an aggressive manner with 5-year survival as low as 30% in non-functioning PNETs [1].
Local disease can be treated surgically, and octreotide has benefits in terms of symptomatic control as well as an antitumor effect. However, for advanced disease, the response to standard chemotherapeutic regimens remains less than ideal. As a greater understanding of the tumor biology of PNETs is taking place, agents targeted at the receptors overexpressed in these tumors are beginning to show some promise. Agents targeted at epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and mammalian target of rapamycin (mTOR), or combinations of targeted agents with cytotoxic chemotherapy appear to play a role in controlling the disease.
This review will address five abstracts, which were presented at the 2010 American Society of Clinical Oncology (ASCO) Annual Meeting (Table 1).

What We Knew Prior to the 2010 ASCO Annual Meeting
Pancreatic neuroendocrine tumors have long been felt to be rare as far as pancreatic neoplasms are concerned. Surveillance, Epidemiology and End Results (SEER) data reports an incidence of 1.4%, with a far better overall prognosis and long term survival as compared to cancer arising from the exocrine pancreas [2]. Interestingly, autopsy studies suggest that although not clinically apparent, the incidence may actually be higher; up to 10% [3].
A recent epidemiological study reports a greater prevalence of neuroendocrine tumors than previously reported. Using SEER data from 1973-2004, a significant increase in age-adjusted incidence was found: 1.09 per 100,000 inhabitants in 1973 to 5.25 per 100,000 inhabitants in 2004. For those tumors which originate in the pancreas, the incidence was reported to be 0.32 per 100,000 from 2000-2004, with a median age of 60 years at diagnosis. These tumors are generally felt to be more slow growing and indolent than other malignancies, but in the analysis only 14% of patients presented with localized disease, 22% with regional involvement and 64% with distant metastases [4].
PNETs may either be hormone producing (such as: insulin, 17%; gastrin, 15%; VIP, 2%; glucagon, 1%; serotonin, 1%; somatostatin, 1%), or can be nonfunctional and as a result often detected later in their course as they present with symptoms of mass effect rather than hormone production [3]. WHO classifications separate these tumors into welldifferentiated endocrine tumors with either benign or uncertain behavior (depending on size and proliferation indices), well-differentiated endocrine carcinoma, and poorly-differentiated endocrine carcinoma. The majority of pancreatic neuroendocrine tumors are sporadic, but they can be associated with genetic disorders including MEN1, von Hippel-Lindau Disease, neurofibromatosis 1, and tuberous sclerosis. The only curative therapy is surgical resection if possible, but as seen the majority of cases present with evidence of distant metastatic spread. As a result, the issue of how to treat disease in the liver has been addressed, with surgical resection, transcatheter arterial chemoembolization, and radiofrequency ablation as viable options. Liver transplantation has even been considered in a select subset of patients with unresectable hepatic disease [5].
Standard medical therapy aims to treat symptoms of these tumors with somatostatin analogues or interferon alpha. Somatostatin analogues result not only in the palliation of symptoms, thereby improving the quality of life [6], but the PROMID study published in 2009 also demonstrated that Sandostatin LAR® (Novartis International AG, Basel, Switzerland) increases the time to tumor progression as compared to placebo in both functionally active and inactive tumors of midgut origin (jejunum, ileum, appendix and proximal colon). The primary endpoint of time to tumor progression was found to be 14.3 months in the octreotide LAR® group compared to 6 months in the placebo group. Tumor response was a secondary outcome, with stable disease in 66.7% of patients receiving octreotide LAR® as compared to 37.2% receiving placebo. Additionally, a surgically resected primary tumor and low hepatic tumor burden (defined as equal to, or less than, 10%) seemed to confer the greatest benefit [7]. Therefore, octreotide remains the mainstay of treatment for these tumors.
Once disease progresses, chemotherapy can be utilized, although with mixed results. Streptozosin, adriamycin, 5-FU and dacarbazine have been used both as single agents and in combination, with streptozosin/ doxorubicin as the recommended regimen [8].
Well-differentiated pancreatic neuroendocrine tumors have been found to have a poor response to chemotherapy as compared to poorly differentiated tumors. This is thought to be related to low mitotic rates (majority of patients with Ki 67 of less than 2% in the PROMID study), high levels of bcl-2 and higher expression of the multidrug resistance gene [9]. A study of cisplatin/etoposide was associated with a 67% response rate for poorly differentiated tumors with little activity in well-differentiated tumors, making this an option for those less differentiated cases [10].
Temozolomide has been studied as an option based on the activity seen with dacarbazine, as they share an active metabolite. Responses have been reported for PNET tumors with lower levels of O-6-methylguanine- DNA methyltransferase (MGMT) expression with one study describing deficiency of MGMT expression in 51% of pancreatic neuroendocrine tumor samples, and 34% of these demonstrating a partial or complete response to temozolomide based regimens [11]. Temozolomide alone has been studied with unclear efficacy, but in combination with capecitabine or bevacizumab has shown promise.
More recently, the focus has been on targeted agents to treat this widely variable disease.
Pancreatic neuroendocrine tumors have been shown to have increased expression of several receptors, including those for EGF, PDGF, insulin-like growth factor (IGF)-1 and VEGF. Sunitinib, which works to inhibit VEGF-1, 2 and 3 and PDGF, has been evaluated in a phase II trial where 107 patients received sunitinib at 50 mg/day for 4 weeks on, 2 weeks off. Response rates overall for pancreatic endocrine tumors were 17% with 68% of patients demonstrating stable disease. Overall response rate was 2.4% for carcinoid patients with 83% demonstrating stable disease. While the authors concluded that there was antitumor activity in pancreatic neuroendocrine tumors, activity in carcinoid tumors could not be determined [12]. Updated data from the phase III trial comparing sunitinib to placebo was presented at the ASCO Annual Meeting of this year. This study ended early due to the superiority of sunitinib arm to the placebo arm.
The intracellular protein kinase mTOR mediates cell signaling downstream through multiple signaling pathways including IGF-1, EGF and VEGF. The mTOR inhibitor everolimus has been shown to have activity in a variety of solid tumors in vivo. A phase II study evaluated everolimus (5 mg or 10 mg daily in combination with octreotide LAR® at a dosage of 30 mg every 28 days) for low to intermediate grade pancreatic neuroendocrine tumors and showed promising activity with a 22% partial response rate, 42% of patients had stable disease and there was a 60- week median progression free survival. Prior studies with octreotide did not demonstrate an affect on progression free survival [13].
The subsequent phase II RADIANT-1 trial randomized 160 patients with metastatic pancreatic neuroendocrine tumors, who received prior chemotherapy and had disease progression, to either everolimus 10 mg per day alone (115 patients) or in combination with octreotide LAR® (45 patients). An objective partial response rate of 9.6% was seen, with stable disease in 67.8% in the everolimus group. The combined group demonstrated a partial response rate of 4.4% with 80% of patients having stable disease. The combined group had a progression free survival of 16.7 months while the everolimus alone group had a progression free survival of 9.7 months. These data help support the conclusion that everolimus has antitumor activity in patients with disease progression after receiving prior chemotherapy [14].
What We Learned at the 2010 ASCO Annual Meeting
Three abstracts discussed sunitinib as a treatment option for pancreatic neuroendocrine tumors. A study by Niccoli et al. (Abstract #4000) [15] presented sunitinib as a treatment for advanced, welldifferentiated pancreatic endocrine tumors. Raymond et al. (Abstract #4031) [16] found this agent to be of benefit and this benefit to be independent of baseline characteristics. Vinik et al. (Abstract #4003) [17] found that not only is sunitinib an active treatment, but it does so without sacrificing quality of life.
Sunitinib
Abstract #4000: Results of a phase III trial of sunitinib versus placebo [15]
This study ended early based on significant improvement in progression free survival, overall survival, and objective response rate in the sunitinib arm compared to placebo control arm. The trial enrolled 171 patients with well differentiated PNET with disease progression in the prior year and they were randomized to receive sunitinib 37.5 mg/day (86 patients) or placebo (85 patients). Overall, 95% of patients had distant metastases, 89% had prior surgery, and about half received prior chemotherapy (52% in the sunitinib arm, 59% in the placebo arm). Approximately 25% of patients received prior somatostatin analogs (24% sunitinib arm, 22% placebo arm). In this study, 49% of patients had functioning tumors.
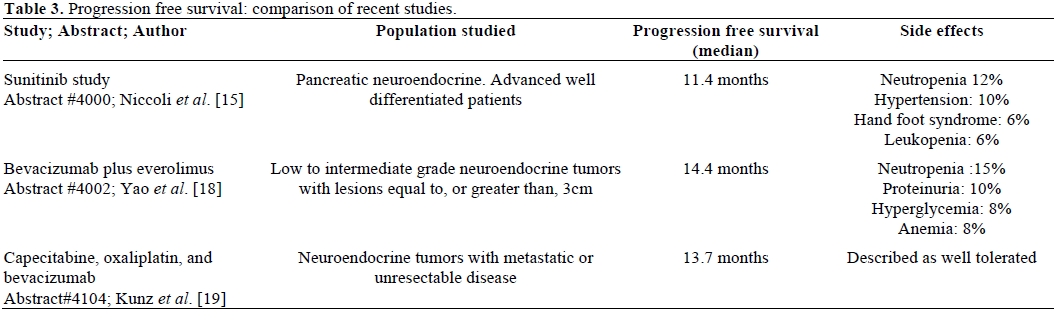
Because the study was stopped early, median overall survival was not reached. However, median progression free survival was found to be significantly longer in the group treated with sunitinib (11.4 months versus 5.5 months) with fewer adverse events (Table 2).
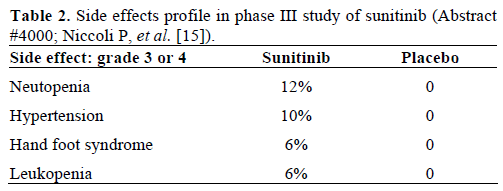
Abstract #4003: Patient reported tolerability of sunitinib in phase III study [17]
This group of investigators used a quality of life questionnaire to study how patients in the above study tolerated treatment. Overall, 73 out of 86 patients in the sunitinib group and 71 out of 85 patients in the placebo group were evaluable. Data were obtained on day 1 of every 4 week cycle, and data from the first 10 cycles were analyzed. Although diarrhea and insomnia seemed to be statistically worse in the treatment group, quality of life scores did not show clinical or statistically significant differences. Thus, sunitinib appears to be a viable treatment option in terms of patient tolerability.
Abstract #4031: Subgroup analysis of sunitinib phase III trial [16]
This study sought to determine if there were certain patient characteristics, which might predict a better response to sunitinib therapy. Raymond et al. found that this was not the case. The parameters evaluated were age (less than 65 years versus more than, or equal to, 65 years), race (Caucasian or not), gender, performance status (Eastern Cooperative Oncology Group (ECOG) 0 compared to 1 and 2), number of sites of metastatic disease (2 or less versus 3 or more), and time from diagnosis to enrollment in study (more than 3 years versus 3 or less). All groups benefited in terms of progression free survival. Prior therapy did not have an effect on response to treatment. For the analysis, 72 patients had Ki-67 values available, and for those with Ki-67 index equal to, or less than 5%, there was a progression free survival improvement with a hazard ratio (HR) of 0.378 (P=0.0259). The conclusion that can be drawn is that sunitinib is a viable treatment option in all patients with advanced well-differentiated pancreatic neuroendocrine tumors.
Targeted Agents: mTOR plus VEGF Inhibitors
Abstract #4002: Bevacizumab plus everolimus in NET [18]
Yao et al. presented data on a study looking at the combination of the mTOR inhibitor everolimus with the VEGF inhibitor bevacizumab. Functional CT was used to assess for changes in tumor blood flow, blood volume, mean transit time and permeability. This study demonstrated an antitumor effect of the combination of both targeted agents, and also demonstrated a correlation between disease response and functional CT findings.
Overall, 39 patients were studied and randomized to either bevacizumab or everolimus for 21-day cycles with the other agent added on with cycle 2. Partial responses were seen in 26%, stable disease in 69% and 3% had disease progression. A correlation was noted with functional CT: disease responses correlated to greater decreases in blood flow, blood volume, and higher mean transit time increases as well as higher permeability and higher post-treatment mean transit time.
The addition of everolimus enhanced the decrease in tumor blood flow seen with bevacizumab alone, and the combination resulted in disease responses.
Cytotoxic Chemotherapy: Still a Viable Option?
Abstract #4104: Capecitabine, oxaliplatin, and bevacizumab for advanced NET [19]
Although the focus of recent investigations has favored targeted agents, a phase II study was presented evaluating cytotoxic chemotherapy in combination with the targeted agent, bevacizumab. Forty patients with advanced neuroendocrine tumors received capecitabine (850 mg/m2 bid for two weeks of a three week cycle) and oxaliplatin (130 mg/m2) in combination with bevacizumab (7.5 mg/kg i.v.). Among the 31 patients for whom responses could be assessed, 7 patients (23%) demonstrated a partial response. Two patients (6%) progressed, and the majority of patients (71%) demonstrated stable disease. Twenty patients randomized had pancreatic neuroendocrine tumors, and 6 out of the 7 partial responses were in patients with pancreatic tumors. Median progression free survival was 13.7 months (Table 3).
In summary, pancreatic neuroendocrine tumors are generally felt to be indolent, although the majority do present at an advanced stage. In the past, treatment options have been limited, with hormonal treatment with octreotide as the primary therapeutic approach. Chemotherapeutic agents have been used with limited efficacy (less effective in well-differentiated tumors). A recently completed phase II trial supports the combination of capecitabine, oxaliplatin and bevacizumab in advanced disease. Targeted therapyhas a clear role as these tumors do overexpress receptors for EGF, PDGF, IGF-1, and VEGF. Sunitinib has been established as a potential treatment option and the phase III data support its benefit in terms of progression free survival without sacrificing quality of life. The mTOR inhibitors have been shown to have activity alone or in combination with somatostatin in phase II studies. The combination of an mTOR inhibitor and a VEGF inhibitor also had promising results.
Conflict of interest The authors have no potential conflicts of interest