- (2010) Volume 11, Issue 4
Bryan W Chang1, Eduardo Siccion2, Muhammad Wasif Saif1
1Yale Cancer Center, Yale University School of Medicine. New Haven, CT, USA.
2University of Connecticut Health Center. Farmington, CT, USA
Pancreatic cancer is the 4th leading cancer cause mortality in both men and women. Pancreatic cancer is usually diagnosed in the advanced setting, and only 10-15% of patients present with operable disease. About 25% are locally advanced and unresectable and the rest are metastatic. Studies presented at the 2010 American Society of Clinical Oncology (ASCO) Annual Meeting highlighted both current treatment options and promising novel therapeutic agents and approaches.
capecitabine; gemcitabine; Pancreatic Neoplasms; Radiotherapy; Receptors, Urokinase Plasminogen Activator; sorafenib
ASCO: American Society of Clinical Oncology; ECOG: Eastern Cooperative Oncology Group; uPA: urokinasetype plasminogen activator
What We Knew before ASCO 2010
There has been modest improvements in the median survival of patients with locally advanced pancreatic cancer. Improved survival with chemoradiation compared to radiation alone was demonstrated in the 1981 Gastrointestinal Tumor Study Group (GITSG) study with a median survival of 10 months for patients treated with combined modality therapy. The recently completed Eastern Cooperative Oncology Group (ECOG) 4201 study was under-enrolled but showed a median survival of 11.2 months for patients receiving chemoradiation versus 9 months for patients receiving gemcitabine alone.
What We Learned at ASC0 2010
Several of the studies presented focused on treating locally advanced pancreatic cancer with chemotherapy alone, while others addressed the use of novel chemotherapeutic or targeted agents in combination with radiation therapy and chemotherapy (Table 1).
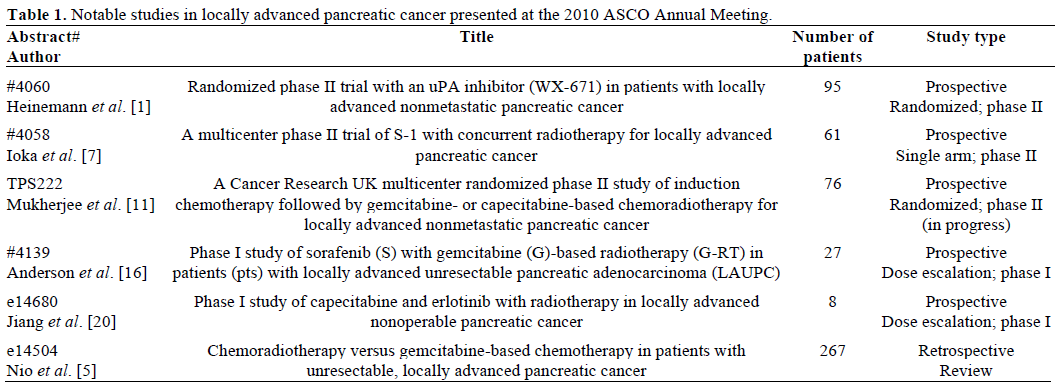
Treatment of Locally Advanced Disease with Chemoradiation versus Chemotherapy Alone
Abstract #4060. Randomized phase II trial with an uPA inhibitor (WX-671) in patients with locally advanced nonmetastatic pancreatic cancer [1]
As an orally available prodrug of WX-UK1, WX-671 exhibits inhibition of urokinase-type plasminogen activator (uPA) which reduces baseline pancreatic cancer cell invasion [2]. As shown by Ertongur et al. [3], WX-UK1 demonstrated that this compound inhibited in vitro tumour cell matrigel invasion by a variety of human cancer cell lines. WX-UK1, a derivative of 3-aminophenylalanine in the Lconformation with inhibitory antiproteolytic properties, selectively inhibited tumour-related proteases from rats and humans and when administered for 35 days it impaired primary tumour growth and metastasis of BN472 rat breast cancer in a dose-dependent manner (minimum inhibitory dosage: 0.15 and 0.3 mg/kg/day) [4]. Ninety-five patients with locally advanced unresectable pancreatic cancer were randomized to three cohorts receiving WX-671 at 0, 200 mg and 400 mg daily oral doses and weekly gemcitabine 1,000 mg/m2 i.v. each [1]. The results showed an increase in overall survival from 10.2 months (gemcitabine alone) to 13.5 months for the combination of gemcitabine and WX-671. One-year survival increased from 37% with gemcitabine to 53% when combined with 400 mg WX- 671.
Abstract e14504. Chemoradiotherapy versus gemcitabine-based chemotherapy in patients with unresectable, locally advanced pancreatic cancer [5]
Nio, et al. reviewed 267 patients with locally advanced pancreatic cancer who received chemoradiotherapy vs. gemcitabine based chemotherapy as first line therapy [5]. Their results showed a no significant differences in long term outcomes with the two groups. Discussion. This study is retrospective and some patients were treated with non-standard regimens, such as gemcitabine with S-1 and S-1 with radiation. The overall survival figures are on the high side for both arms. It is not clear whether induction chemotherapy was used before chemoradiation, or whether maintenance chemotherapy was given equally in both arms. ECOG E4201 utilized gemcitabine-based chemotherapy and modern radiation techniques and doses. The study was underpowered, but there was a significant survival benefit for chemoradiation. Data from a larger Japanese randomized study addressing this question is pending [6].
Novel Radiosensitizers
Abstract #4058. A multicenter phase II trial of S-1 with concurrent radiotherapy for locally advanced pancreatic cancer [7]
S-1 (TS-1®, Taiho Pharmaceutical, Tokyo, Japan) is an orally active combination of tegafur (a prodrug that is converted by cells to fluorouracil), gimeracil (an inhibitor of dihydropyrimidine dehydrogenase, which degrades fluorouracil), and oteracil (which inhibits the phosphorylation of fluorouracil in the gastrointestinal tract, thereby reducing the gastrointestinal toxic effects of fluorouracil) in a molar ratio of 1:0.4:1 [8]. It has been recently shown to be marginally effective and well tolerated in the second-line setting in patients with gemcitabine-refractory advanced pancreatic cancer as a monotherapy [9]. Ioka et al. conducted a phase II study on 61 chemo-naïve patients with locally advanced pancreatic cancer treated with oral daily doses of S-1 80 mg/m2 bid during radiation therapy and continued with a maintenance dose (80 mg/m2/day for 28 days with a 2 week rest) [7]. Radiation therapy consisted of 50.4 Gy delivered in 28 fractions with no elective nodal irradiation. Their results showed median values of progression free survival of 9.7 months and of overall survival of 16.2 months and a 1-year survival of 71.7%. Elevated pretreatment levels of CA 19-9 greater than 100 U/mL decreased by 50% in 34 out of 42 patients. Grade 3-4 toxicity included leucopenia in 6 patients and anorexia in 4 patients. One patient died of duodenal and biliary perforation.
Discussion. This single-arm study yielded a promising median survival of 16.2 months with an acceptably low level of toxicity. As there is evidence that S-1 is metabolized differently in non-Asian patients [10], further study in non-Asian populations is warranted. It would also be interesting to consider integration of S-1 into a gemcitabine-based regimen, although this would undoubtedly add to toxicity. Overall, this data appears very promising.
Abstract TPS222. A Cancer Research UK multicenter randomized phase II study of induction chemotherapy followed by gemcitabine- or capecitabine-based chemoradiotherapy for locally advanced nonmetastatic pancreatic cancer [11]
The combination of gemcitabine, capecitabine and radiation therapy in the adjuvant setting has been shown to be relatively active and a well tolerated regimen with a median progression free survival of 21.7 months and median overall survival of 45.9 months [12]. Mukherjee et al. are conducting a Cancer Research UK funded National Cancer Research Institute study using a two-arm randomized phase II trial [11]. Of the patients who respond or who have stable disease after 16 weeks of GemCap induction chemotherapy, 76 will be randomized to 5.5 weeks of consolidation radiation therapy consisting of 50.4 Gy in 28 fractions with either gemcitabine 300 mg/m2 weekly or capecitabine 830 mg/m2 bid (Figure 1).
Blood samples from the trial will be stored for future translational studies.
Discussion. Both capecitabine [13] and gemcitabine have shown promise as radiosensitizing agents in locally advanced pancreatic cancer [6]. The use of a period of induction chemotherapy prior to chemoradiation has been proposed as a way of selecting patients with favorable disease biology for more aggressive local therapy, but this approach is supported only by retrospective data [14]. This study is useful in that it will provide a prospective evaluation of the use of induction chemotherapy, and in that capecitabine may represent a more tolerable oral alternative to gemcitabine for radiosensitization [15].
Addition of Biological Agents to Chemoradiation
Abstract #4139. Phase I study of sorafenib (S) with gemcitabine (G)-based radiotherapy (G-RT) in patients (pts) with locally advanced unresectable pancreatic adenocarcinoma (LAUPC) [16]
Around 90% of pancreatic cancer (PC) carries the ras oncogene mutation, leading to constitutive activation of the Ras-Raf-MAPK signal transduction pathway. Sorafenib, an oral multi-tyrosine kinase inhibitor of Braf, VEGFR2, and PDGFR-B, has in vitro activity against pancreatic cancer cell lines and xenograft models dependent on mutant Ras activation [17]. Combined with gemcitabine, it resulted in growth delays of pancreatic tumor xenografts. Anderson et al. hypothesized that combining sorafenib with radiation might improve patient outcomes, as radiation can induce an increase in VEGF [18] and activate the proto-oncogene serine/threonine kinase Raf [19]. Twenty-seven patients with locally advanced unresected pancreatic cancer were treated with 1,000 mg/m2 i.v. weekly gemcitabine x3 every 4 week/cycle for 1 cycle, followed by weekly 600 mg/m2 with concurrent intensity modulated radiotherapy (IMRT) 50 Gy to the gross tumor volume in 25 fractions. Four cycles of weekly gemcitabine followed. They received 400 mg bid of sorafenib which was dose escalated during radiotherapy in a "3+3" design from 200 mg qd, 400 mg qd and 400 mg bid, with expansion at maximal tolerated dose. Despite an encouraging activity of median progression free survival of 11 months and overall survival 16 months, around 8 patients had increased gastrointestinal toxicity, two patients had hemorrhagic gastropathy, 4 patients had ulcers, one patient had perforated colon diverticuli, and one patient had rectal bleeding. One patient died due to Pseudomonas infection.
Discussion. The toxicities observed in this trial are concerning. While early studies of gemcitabine with radiation showed high levels of in-field gastrointestinal toxicity, subsequent studies have been conducted demonstrating that gemcitabine at 600 or even 1,000 mg/m2 can be safely delivered during radiation therapy. The possibility that sorafenib is responsible for the enhanced toxicity cannot be ignored. Although the survival data is intriguing, further investigation is needed before proceeding to a higher level of clinical testing.
Abstract e14680. Phase I study of capecitabine and erlotinib with radiotherapy in locally advanced nonoperable pancreatic cancer [20]
To test safety and feasibility of the combination of capecitabine and erlotinib with radiotherapy Jiang et al. adopted a "3+3" dose escalation design with the following dose distributions: dose level I: capecitabine 600 mg/m2 bid, erlotinib 100 mg daily; level II: capecitabine 700 mg/m2 bid, erlotinib 100 mg daily; level III: capecitabine 825 mg/m2 bid, erlotinib 100 mg daily; level IV: capecitabine 925 mg/m2 bid, erlotinib 100 mg. Capecitabine and erlotinib were administered daily from Monday through Friday, concurrent with radiotherapy for 50.4 Gy. Three patients have been recruited in level I and 5 patients in level II with one patient requiring dose reduction due to grade 3 nausea and one-week treatment interruption at dose level II.
Discussion. This study adds to the small but growing body of literature on erlotinib as a radiosensitizing agent in pancreatic cancer [21]. While preliminary data has in general been moderately promising, it is noteworthy that radiation, capecitabine, and erlotinib all have the potential to cause significant (and potentially additive) gastrointestinal toxicity.
The 2010 ASCO Annual Meeting highlighted certain studies in locally advanced pancreatic cancer that are in its early phase of accrual and some completed ones with meaningful results. Although some of the studies reviewed above have shown significant increases in overall survival, none of these regimens can be considered ready for testing in the phase III cooperative group setting. The role of systemic therapy and radiotherapy with its different timing, dosing, and scheduling in locally advanced pancreatic cancer still needs to be defined. Evolving novel therapeutic agents like the uPA inhibitor WX-671 have demonstrated promising results in its early phase studies and further validates the potential benefit of identifying and exploiting new therapeutic targets in this disease. The conference also highlights the growing need to collect tissue specimens for further translational studies. Even though there are still unanswered questions on the specific combined modality that will have an impact on locally advanced pancreatic cancer, it cannot be denied that results from these studies will contribute to better therapies that will improve survival.
Conflict of interest The authors have no potential conflicts of interest