- (2014) Volume 15, Issue 6
Mohammad Taghi Mohammadi1, Leila Pirmoradi1, Fakhrodin Mesbah2, Akbar Safaee3 and Gholam Abbas Dehghani1
Departments of 1Physiology, 2Anatomical Sciences and 3Pathology, Shiraz University of Medical Sciences, Shiraz, Iran
Received August 10th, 2014 – Accepted October 25th, 2014
Context Oral vanadyl sulfate (vanadium) has potent hypoglycemic effects in diabetes animals, but data about its actions on pancreatic beta-cells (BC) ultrastructure is limited. Objective Partial diabetic rats were treated with vanadium and insulin injection and their effects on BC ultrastructure are studied. Methods Male rats were made diabetic with intravenous streptozotocin injection (STZ, 40 mg/kg). Animals were randomly divided to control (CD), vanadium-treated (1 mg/mL VOSO4 + 5H2O in base solution, VTD) and insulintreated (80 U/kg/day NPH insulin injection, ITD) diabetic groups. Treatments started 10 days after STZ injection and terminated after 2 months. Intermittent tail blood samples were taken for measurements of blood glucose (BG) and plasma insulin (PI). Finally animals were sacrificed and pancreata prepared for assessments of BC ultrastructure, islets histology and insulin-immunoreactivity (IIR). Results Vanadium decreased BG (p0.0001), elevated the redced PI (p0.001), prevented islet atrophy ad restored BC ultrastructure. Low BGseen during treatment in VTD and ITD only persisted in VTD after vanadium withdrawal. Hyperglycemia worsened in CD and repaired in ITD shortly after insulin withdrawal. CD islets were atrophied, had scattered IIR spots. BC had pyknotic nuclei, vacuolated cytoplasm and few tiny insulin secretory granules. VTD islets looked normal with compact centered IIR spots. BC had well-developed endoplasmic reticulum, many insulin secretory granules and mitochondria. ITD islet structure was slightly better than CD and BC had some immature insulin secretory granules. Conclusion The trophic actions of vanadium in diabetic rats effectively renovated betacell ultrastructure and prevented pancreatic islets atrophy, whereas the relief of glucotoxicity seen with insulin treatment could repair some beta cells and partially prevented islet atrophy.
Vanadium; Diabetes Mellitus, Experimental; Pancreas; Insulinoma; ultrastructure [Subheading]
Clinical trials and laboratory experiments have showed vanadium therapy reduces high blood glucose levels in type 1 and 2 diabetic patients and streptozotocin-induced diabetic animals [1-5], whereas continuous stimulations of pancreatic islets, seen during chronic hyperglycemia provoke beta-cell and dysfunction apoptosis [6-8].
It is strongly believed that vanadium might be a valuable supplement to insulin in the treatment of diabetes mellitus [4, 5, 9, 10]. Many reports have reiterated in partial diabetic rats vanadium reduced high blood glucose, proliferated pancreatic beta cells and prevented the recurrence of hyperglycemia after withdrawal [11-13]. Microscopic examinations of the pancreas of diabetic rats have also demonstrated vanadium repaired the damaged beta cells, prevents pancreatic islet atrophy and restored islet insulin storage [5, 13-15]. With appreciation of the beneficial actions of vanadium in relieving diabetic symptoms and preventing islet-dysfunction, the present study intended to find whether the relief of hyperglycemia with vanadium or insulin therapy helps the repairing of beta-cell ultrastructure.
Male healthy Sprague Dawley rats (200-250 g) were obtained from Central Animal House Facility of Shiraz University of Medical Sciences. All the protocols of the study were approved by the Institutional Ethical Committee of the University which follows the NIH guidelines for care and use of animals (NIH publication No. 85-23, revised in 1996). Rats were housed in standard cages in a room with controlled temperature (22-24°C), humidity (40-60%) and light period (07.00-19.00), while having full access to food (rat food, Parsdam; Tehran, Iran) and water.
Drinking Solutions
The drinking solutions used in this study contained 3 g/L NaCl distilled water (base solution) or vanadyl sulfate (1 mg/ml VOSO4 + 5 H2O, Merck, Germany) in base solution. The solutions were prepared fresh every 3-5 days and stored in the dark cold room (4oC) until use.
Blood Samples
Rats were anesthetized with ether and blood (500 μL) was collected from the tip of the snipped tail. Two μL of the blood was used to measure blood glucose (BG) with glucose monitoring system (Glucocard® 01-Mini, Arkray, Inc., Kyoto, Japan) and the rest was centrifuged (12,000 ×g) and its serum were stored in a freezer (-70°C) for the future assessments of insulin.
Induction of Diabetes
Diabetes was induced with a single intravenous dose of freshly prepared streptozotocin (STZ; 40 mg/kg dissolved in normal saline, Sigma, USA) through the lateral tail vein. Non-diabetic rats received the same volume of normal saline. Induction of diabetes was confirmed 48-72 hours after STZ injection by the presence of hyperglycemia (BG ≥350 mg/dL), polydipsia (fluid intake ≥ 100 mL/day) and polyuria (wet cage). Animals were housed in the same room but in separate cages (1 per cage).
Rats were divided into 4 groups:
The first group (n=12) considered as control normal (CN) which during the study used base solution as drinking water.
The second group (n=9) used as control diabetic (CD) like CN used base solution.
The third group (n=11) considered vanadium-treated diabetic (VTD) used vanadyl solution at concentrations mentioned above.
The fourth group (n=10), served as insulin-treated diabetic (ITD) rats and received daily intraperitoneal injection of NPH (neutral protamine Hagedorn) insulin to bring the levels of blood glucose to VTD group level and used base solution as drinking water. Vanadium or insulin therapy started at 10th day of STZ injection and continued for 60 days.
After 2 months treatments the animals were sacrificed under deep anaesthesia (ketamine/xylazine 70/10 mg/ kg), sacrificed and the whole pancreas was gently dissected out and prepared for staining. The prepared pancreatic tissues were randomly assigned for hematoxylin eosin (H/E), immunohistochemistry. Some the pancreases were prepared for subcellular examinations of beta cells with transmission electron microscope (TEM). Samples were obtained from the same regions of the pancreas to have better judgments about the changes that may occur in islets and their beta cells.
Pancreas was fixed in 10% neutral buffered formalin (pH 7.4) and 48 hours were kept at room temperature. After fixation and dehydration with graded ethanol series, paraffin blocks were cut to 4 microns thick sections, deparaffinized with Xylene and rehydrated, in a descending graded series of ethanol and at the final stage rinsed with 0.02 M phosphate buffer saline (PBS). Three to 5 tissue sections of each pancreas were used for beta cell immunofluorescence staining. At this stage the activity of endogenous peroxidases were blocked by submerging the paraffin-embedded tissues in methanol containing 3% hydrogen peroxide. The use of non-immune serum helped to eliminate non-specific background colors. Primary anti-insulin clone antibody (Sigma I-2018) was used to detect the insulin containing beta cells. For 20 minute the sections successively incubated in biotinylated antibody and rinsed with PBS. Histostain-Plus kit (Zymed Code 859943) was used according to the methods describe by Bolkent et al. investigators [11]. The use of amino ethyl carbazole (AEC) created an intense red deposit around the antigen/antibody enzyme complex. Sections arbitrarily coded and blindly examined with light microscope by two independent experts.
Islets prepared by collagenase according the Lacy method [16]. In brief, the common bile duct was cannulated and the pancreas was distended with 10 ml Hank's Balanced Salt Solution (HBSS). The pancreas was carefully dissected from surrounding tissues, cut into small pieces and immediately digested in HBSS containing 2 mg/mL type B collagenase. Twenty mL of the fresh PBS was added to the digested solution and centrifuged for 5 min (1000 rpm). The supernatants were discarded, the pellets were washed with HBBS and then re-suspended in 15 ml HBSS. The prepared samples were transferred to a 15 ml dark stained Petri dish, islets handpicked under dissecting microscope and subsequently fixed (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer) and finally post-fixed (90 min) in osmium buffer (1% OsO4 in 0.1 M cacodylate). Samples were dehydrated by passing them through an ethanol series, then embedded in Epon (TAAB, Berks, UK) and sectioned into semi-thin (1 μm) pieces. The sections were stained with toluidine blue and examined with light microscopy for documentation of islets. The tissue blocks were re-trimmed and sectioned ultrathin (60-90 nm, collected on copper grids) and immersed in Uranyl acetate (15 min at room temperature). The stained sections were repeatedly washed in distilled water, dried and subsequently stained for 12 min with lead citrate. At the end sections were randomly coded numerically and examined by two individual experts (blinded to the data) with transmission electron microscope (TEM, Philips CM-10).
Data are presented as mean ± SEM. Wilcoxon-Newman and Mannwitny tests were used to obtain the statistical differences among the means of intra and between groups for blood glucose and insulin. Repeated measurement test was used for the comparison of body weight. Values are considered as statistically significant at P < 0.05.
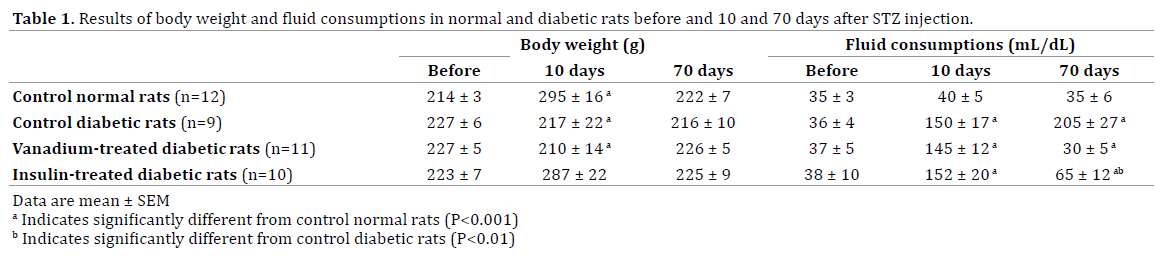
Body Weight and Fluid Consumptions
Table 1 shows the growth body weight and daily fluid consumptions of normal and diabetic groups before and after induction of diabetes. While, the growth weight in CD and VTD were stopped a steady state increase in body weight was observed in CN and ITD groups. The measurements of fluid consumption indicated induction of diabetes significantly increased daily water intake (Table 1). Tow month treatment of diabetic rats with vanadium or insulin significantly reduced water intake such there were not any significant differences among data of CN and VTN groups.
Blood Glucose and Insulin Levels
The BG of normal and diabetic groups is presented in Figure 1. Data indicated before the start of the treatment BG significantly increased in all diabetic rats of CD, VTD and ITD groups. During 2 month period of the study hyperglycemia worsened in CD group, but significant reductions were observed in BG of VTD (138 ± 14 mg/dL) and ITD (141 ± 14 mg/dL).
Figure 1. The level of blood glucose in the control normal(CN), control diabetic (CD), vanadium-treated(VTD) and insulintreated (ITD) diabetic groups. Data expressed as mean ± SEM. +Significantly different from CN and their own pretreated conditions CN (p<0.0001). !Significantly different from CD (p< 0.01).
Figure 2 shows the levels of insulin in normal and diabetic rats. Before induction of diabetes no significant variations were observed among the levels of insulin. Thirty days after the start of treatments (40 days after STZ injection) significant, but similar, reductions were observed in the levels insulin of both CD and VTD groups. After 2 month vanadium therapy, compared to CD group, the reduced insulin levels, seen in CD) was significantly increased insulin in VTD rats. However, at this stage the increased level of insulin seen in VTD was significantly lower than CN.
Figure 2. The level of insulin in the control normal(CN), control diabetic (CD), vanadium-treated(VTD) and insulin-treated (ITD) diabetic groups. Data expressed as mean ± SEM. + Significantly different from CN and their own pretreated conditions CN (p<0.0001). !Significantly different from CD (p< 0.01).
Figures 3 and 4 represent the photomicrographs of H/E and toluidine staining of the pancreatic tissues of normal and diabetic rats. The sections of the pancreatic tissues of normal rats showed regular islets with clustered of beta cells placed centrally (Figures 3-CN and 4-CN), whereas in CD rats the islets were degenerated, necrotic an atrophied and beta cells (Figures 3-CD and 4-CD), had pyknotic nuclei with dark eosinophilic cytoplasm. The islets in VTD, compared to CD, had less degenerated beta cells and in some cases they almost looked normal in size and well granulated beta cells (Figures 3-VTD and 4-VTD). The reduced high blood glucose in ITD apparently protected some of the damaged beta cells from glucotoxicity, but in general the shape of ITD islets were far away from VTN or CN groups (Figures 3-VTD and 4-VTD). Overall, most of the beta cells had pyknotic nucleoli with light hydropic degeneration and necrotic (Figures 3-ITD and 4-ITD).
Figure 4. Immunohistochemical staining of the pancreatic islets of normal and experimental groups. Diabetes was induced with a single i.v. injection of STZ (40 mg/kg). Control normal (CN) and diabetic (CD) rats only received base solution (see Material and Methods). Treatments of the diabetic rats with vanadyl (VTD) or NPH insulin (ITD) started at 10 days after STZ injection. The typical distributions of the beta cells with moderate insulin immunoreactivity are well seen in the CN. A weak insulin immunoreaction was seen in the islets cells of the CD and ITD. This reactivity was highly increased in the pancreatic islets of VTD compared to CD and ITD. (Magnification, 400x).
Immunohistochemical staining of the pancreas of CN group showed isles had strong positive insulin-immunoreactive spots (Figure 5-CN). Whereas islets in CD were atrophied and insulin-immunoreactive spots were dispersed or almost missing (Figure 5-CD), some improvements were seen in the islets’ structure of ITD and their insulinimmunoreactivity (Figure 5-ITD). In the opposite the pancreatic islets in VTD looked almost normal, similar to the islets of CN group, with well centrally condensed insulin-immunoreactive spots (Figure 5-VTD).
Figure 5. Histological photographs of toluidine blue staining of the pancreatic tissues of normal and experimental groups of rats (more details are described under figure 1 or material and methods section). The islets of CN were distinct and had normal looking beta cells. The islets of CD and ITD were atrophied, central cells were degenerated and their nuclei varied in size. The islets in VTD, compared to CD, were larger in size and had more beta cells. The central cellular integrity was near normal. Arrows labels beta cells. (Magnification, 400X).
The electron micrographs of pancreatic beta-cell of CN group shows normal cellular organelles such as nucleus, nucleolus, rough endoplasmic reticulum, mitochondria, Golgi complex and large numbers of insulin secretory granules were distributed throughout the cytoplasm (Figure 6-CN). However, the pancreas of diabetic rats of CD group revealed degenerated beta cells with many nuclear envelopes, vacuolization and ballooning appearances of mitochondria as well as dilated rough endoplasmic reticulum. Beta cells showed nuclear pyknosis, indented nuclear membrane with electron-translucent areas in the cytoplasm and the low numbers of insulin secretory granules (Figure 6-CD). Cristae were severely destroyed, endoplasmic reticulum was dilated, chromatin condensation was defined with invaginated nucleolemma, and beta cells contained less cytoplasm vacuolated with fragmented cytoplasmic organelles (Figure 6-CD).
Figure 6. Ultrastructural observations of the pancreatic beta cells of normal and experimental groups of rats. Transmission electron micrographs of the pancreas of control normal (CN), control diabetic (CD), vanadium-treated diabetic (VTD) and insulin-treated diabetic (ITD) rats. Cellular organelles nucleus (N); nucleolus (Nu); rough endoplasmic reticulum (RER); mitochondria (M); and Golgi complex (GC) are marked. Arrow shows central dense core atypical insulin secretory granules (ISG). Beta cells in CN islets contain numerous ISG and welldistinguished central core (arrow). The cellular integrity of beta cells in CD was normal, their nuclei are migrated, RER is compressed with atypical ISG and devoid of central dense core (arrow). Beta cells in VTD are like CN, with abundant typical ISG and central dense core. The nuclei of beta cells in ITD are identical to CN, with typical and atypical ISG, less filled core dense (arrow) and fewer mitochondria.
The electron micrograph of the pancreas of VTD, compared to CD, had proliferated beta cells with moderately increased and well organized nuclear structure as well as plentiful insulin secretory granules (Figure 6-VTD). In the beta cells mitochondria were not swollen and cytoplasmic regions were less vacuolated (Figure 6-VTD). Beta-cell nuclei were normal with finely distributed chromatin and distinct reticular nucleolus. The bilaminar nuclear membrane was distinct and had many pores. Some immature beta cells were present which had few central dense core insulin secretory granules with many Golgi complexes, and beta cells had round elongated mitochondria with distinct transverse cristae (Figure 6-VTD).
Treatment of diabetic rats with NPH insulin somehow protected beta cells from glucotoxicity usually happening with chronic hyperglycemia (Figure 6-ITD). In spite of the fact, seeing some improvements in the ITD islets, these improvements were far away from the islets of CN or VTD groups. In general terms the beta cells in ITD group were much smaller, some had pyknotic nucleoli with marginal hyperchromasia and light hydropic degeneration (Figure 6-ITD).
The result of the present study showed vanadium reversed diabetic states in the majority of the partial STZ-diabetic rats. Vanadium treated diabetic rats remained normoglycemic after vanadium withdrawal until they were killed. Well preserved pancreatic islet beta-cell ultrastructure including normal chromatin distribution, well developed endoplasmic reticulum and well granulated cytoplasm, found in electron microscopic studies of islet beta cells indicated that amelioration of diabetic signs was along with preservation of islet ultrastructure [17].
Our previous study showed that vanadium treatment could reverse diabetic symptoms in partial diabetic rats but failed in severe insulin dependent STZ diabetes animals [12, 18]. The severe diabetic rats needed a minimum amount of intraperitoneal insulin during vanadium treatment to induce normoglycemia but almost in all cases hyperglycemia reoccurred after vanadium or insulin withdrawal [12]. The result of the current study as well as previous reports indicated that vanadium treatment in partial diabetic rats can proliferate beta cells and reverse the signs and symptoms of moderate diabetes without any signs of recurrence of hyperglycemia after vanadium withdrawal [4, 11, 18, 19]. With respect to the presence of normal looking beta cells, abundant insulin secretory granules, and increased concentration of plasma insulin in VTD rats it would be reasonable to conclude besides the insulin mimetic action of vanadium its insulinotropic activity vanadium was the cause of elimination hyperglycemia during vanadium treatment or after withdrawal.
The precise mechanism of vanadium in reversing diabetes in diabetic animals is not clear. Even though vanadium affects the activity of many enzymes involved in the metabolic pathways and mimics the effect of insulin in the peripheral tissues [3, 15], these effects are not sufficient for preventing the reoccurrence of the diabetic symptoms. Compensatory cell regeneration can occur in partial diabetic rats but it cannot preserve normoglycemia in sever diabetic rats [12].
Beta cells have a limited proliferative capacity and cell regeneration can only occur if high blood glucose is reversed shortly after STZ injection [20]. There is some evidence for the presence of beta-cell recovery in the pancreatic islets of ITD rats, but the number, and ultrastructure of the recovered beta cells were far away from normal islet beta cells of CN and VTD rats. The increased number of immature granules and welldeveloped rough endoplasmic reticulum in the islet beta cells of VTD rats led us to propose a role for hyperfunction of the remaining beta cells independent of the alleviation of glucose toxicity happening during hyperglycemia. In this regard vanadium can reverse diabetic symptoms through potentiating the effect of insulin and proliferating normal functioning islet beta cells.
Vanadium treatment can lead to long term reversal of diabetic state in moderate diabetes. The insulin mimetic effects of vanadium make it a suitable element for amelioration of diabetic symptoms in type 2 diabetes which have enough endogenous insulin. The protective or regenerative role of vanadium is more likely independent of the relief of glucose toxicity.
The authors are cordially appreciating the financial support (Grant No. 90-5600) of Vice Chancellor for Research of Shiraz University of Medical Sciences, Shiraz, Iran. Authors cordially appreciate the sincere assistance of Mr. Masood Monjazeb of Metabolism & Research Center of Nemazi Hospital for measuring plasma insulin.
Authors have no conflicts of interest