- (2011) Volume 12, Issue 2
Nektaria Makrilia1, Konstantinos N Syrigos1, Muhammad Wasif Saif2
1Oncology Unit, Third Department of Medicine, Sotiria General Hospital, Athens Medical School. Athens, Greece
2Columbia University College of Physicians and Surgeons and Pancreas Center, Presbyterian Hospital. New York, NY, USA
While gemcitabine-based regimens are currently accepted as the standard first-line treatment of patients with locally advanced or metastatic pancreatic adenocarcinoma, there is no consensus regarding treatment in the second-line setting. This review is an update from the 2011 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium regarding recent developments in the treatment of refractory pancreatic cancer, as these were presented in Abstracts #237 and #272 of the meeting.
gemcitabine; irinotecan; Pancreatic Neoplasms; Treatment Failure
ASCO: American Society of Clinical Oncology; FOLFIRI: irinotecan with 5-FU and folinic acid; FOLFOX: folinic acid plus 5-FU plus oxaliplatin; PEP02: liposome irinotecan
Pancreatic cancer remains the fourth leading cause of cancer-related mortality with an estimated total of 43,100 new cases and 36,800 deaths in 2010 in the USA alone [1]. Overall survival remains poor despite advances in therapeutics. Gemcitabine-based regimens represent the standard systemic first-line treatment in patients with advanced pancreatic cancer, offering a better quality of life as well as a small survival benefit [2]. Only a small percentage of patients who exhibit disease progression after first-line treatment continue to receive second-line therapy, mainly because of poor performance status. Therefore, few randomized trials have been conducted and there is currently no consensus on the standard of care for refractory pancreatic cancer [3].
Oettle et al. [4] evaluated folinic acid plus 5-FU plus oxaliplatin (FOLFOX) as second-line treatment in advanced pancreatic cancer and they were the first to establish that chemotherapy offers better overall survival to refractory patients as compared to best supportive care (21 vs. 10 weeks, P=0.007). According to the final results of the Charité Onkologie trial (CONKO-003), the addition of oxaliplatin to 5-FU and leucovorin improves overall survival and progressionfree survival when compared to 5-FU and leucovorin [5]. Based on the above, it has been suggested that FOLFOX become a standard second-line regimen [6]. Some studies have demonstrated that the doublet of gemcitabine and oxaliplatin can be used as second-line treatment in patients refractory to standard gemcitabine regimen [7, 8]. Activity of oxaliplatin has also been shown in combination with capecitabine after gemcitabine failure [9]. These results were confirmed in a phase II study by Dr. Mane et al., presented at the 2011 ASCO GI Cancer Symposium (Abstract #308) [10], but it should be noted that the latter trial enrolled patients with pancreatic or biliary adenocarcinoma and that results were reported on the total of patients.
Regarding taxanes, paclitaxel monotherapy has been suggested as an additional therapeutic option with considerable efficacy and low toxicity in second-line treatment [11]. A recent retrospective study evaluated docetaxel monotherapy as well as docetaxel-based doublets in the treatment of refractory pancreatic cancer and mild activity was shown with no grade 3 or 4 toxicity [12].
Irinotecan has been evaluated in combination with oxaliplatin in patients with advanced pretreated pancreatic cancer exhibiting modest activity and manageable toxicity [13] and offering median overall survival of 4.1 months [14].
Irinotecan with 5-FU and folinic acid (FOLFIRI) showed notable activity and a good toxicity profile after gemcitabine failure [15, 6].
S-1, an oral fluoropyrimidin, has also been investigated in Japanese populations after failure of gemcitabinebased treatment. It seems that this agent is tolerable and marginally effective, offering median overall survival of 5.8 months [16, 17].
Targeted therapies are also being studied in refractory pancreatic cancer. The combination of bevacizumab and erlotinib was recently evaluated in this setting but despite good tolerability, the results were poor [18], as were the results of the use of everolimus [19] and sunitinib [20] as single agents. Bevacizumab monotherapy or its administration in combination with docetaxel did not show any antitumor activity after gemcitabine failure [21].
With regard to treatment of refractory pancreatic cancer after failure of at least one line of therapy, two important abstracts were presented at the 2011 ASCO GI Cancer Symposium (Table 1). Both evaluated the use of irinotecan-based regimens in this treatment setting.
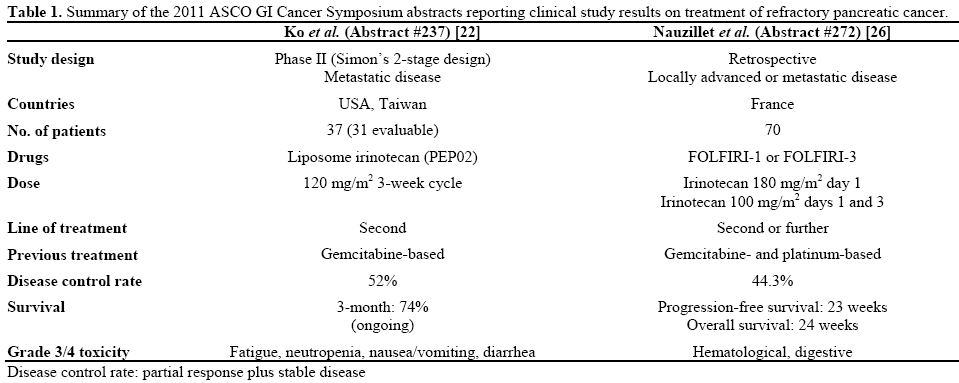
Dr. Ko et al. presented a phase II trial conducted in three centers in the USA and Taiwan (Abstract #237) [22]. They studied the use of single-agent PEP02, a novel nanoparticle liposome formulation of irinotecan, in refractory pancreatic cancer. It is characterized by improved pharmacokinetics and better tumor localization of irinotecan and of its active metabolite SN38, as compared to the free form of the drug. Favorable safety and efficacy of PEP02 were shown in previous phase I studies of patients with refractory solid tumors, such as pancreatic cancer [23, 24]. PEP02 has been studied as monotherapy [23] as well as in combination with 5-FU and leucovorin [24] and tumor response was reported. In the 2011 Ko et al. study [22], 37 patients with metastatic pancreatic cancer received triweekly PEP02 at a dose of 120 mg/m2 as second-line treatment after gemcitabine failure. According to results based on the first 31 evaluable patients, a 52% disease control rate was achieved. CA 19-9 levels decreased more than 50% in one third of patients whose baseline levels were originally elevated. The study met its primary endpoint of 3-month overall survival as the latter reached 74%, with one patient surviving for more than one year. Toxicity was considered acceptable, with fatigue (31%) and neutropenia (25%) being the two most common grade equal to, or greater than, 3 adverse events.
Regimens combining irinotecan with 5-FU and folinic acid (FOLFIRI) have been administered to patients with advanced pancreatic cancer in the first- [25] and second-line setting [15] and data from phase II studies have shown modest efficacy with tolerable toxicity. Gebbia et al. [6] conducted a relevant retrospective study in 40 patients with refractory pancreatic cancer. A new larger retrospective study, conducted in two French institutions, was presented by Dr. Neuzillet et al. at the 2011 ASCO GI Cancer Symposium reporting on the use of FOLFIRI after one or more lines of treatment (Abstract #272) [26]. It included 70 patients with unresectable, locally advanced or metastatic, pancreatic cancer with an overall maspin score less than 3. These patients had previously received gemcitabine and platinum-based chemotherapies. Approximately one third of patients had been administered one prior regimen, 57% had received two lines of treatment and only 8.8% had received three or more lines. Sixty of 70 patients (85.7%) received FOLFIRI-1 (irinotecan 180 mg/m2 day 1) and the rest were administered FOLFIRI-3 (irinotecan 100 mg/m2 days 1 and 3). Disease control rate was 44.3%. Oneyear and two-year progression-free survival was 17% and 3%, respectively, whereas overall survival rates were 24% and 9%, respectively. Dosage adjustment was necessary in 21 patients (30%) and adverse events were considered tolerable with no toxic deaths reported.
Very few options are available for patients with advanced pancreatic cancer after failure of gemcitabine-based regimens. Irinotecan monotherapy has already been evaluated in patients treated with first-line gemcitabine-based chemotherapy: in 2009, Yi et al. [27] reported the results of a phase II trial evaluating biweekly doses of irinotecan monotherapy (150 mg/m2) as salvage treatment in this setting. However, Ko et al. [22] presented the first phase II study of a novel liposomal irinotecan formulation in the second-line treatment of these patients. In both trials, disease control rates were comparable (48 vs. 52% in the Yi and Ko studies, respectively) as were the percentages of patients that exhibited more than 50% decrease in their CA 19-9 levels (33% in both studies). In terms of survival, three-month overall survival seems considerably higher in the liposomal irinotecan study according to the preliminary data presented at the 2011 ASCO GI Cancer Symposium (74% vs. approximately 40% in the Yi et al. trial). However, it should be noted that with regard to toxicity, the liposomal formulation of irinotecan seems to be associated with a significant greater percentage of grade 3/4 adverse events. In this study, fatigue grade equal to, or greater than, 3 is reported in 31% of patients whereas in the Yi et al. study this adverse event was not reported. This difference in toxicity needs to be taken into account as treatment in the second-line setting is often palliative and one of its main objectives is maintaining quality of life.
FOLFIRI regimens have been studied in the past in the treatment of gemcitabine refractory pancreatic cancer. The Yoo et al. [15] phase II study was the first to show favorable efficacy and toxicity profile in gemcitabine pretreated patients. Gebbia et al. [6] retrospectively examined 40 patients who received standard biweekly FOLFIRI after gemcitabine failure and suggested this regimen be used selectively in patients with good performance status or good response to first-line treatment. The 2011 Neuzillet et al. [26] study was also retrospective and showed comparable efficacy results (50% vs. 44.3% disease control rates in the Gebbia et al. and Neuzillet et al. studies, respectively). Estimated median overall survival was 6 months in both studies and toxicity was mainly hematological and gastrointestinal. The Neuzillet et al. trial is the first study to report considerable efficacy and manageable toxicity in patients receiving third- and further-line of chemotherapy. However, what needs to be noted is that patients included were of significantly better performance status (42.9% had performance status equal to 0 vs. 15% and 0.5% in the Yoo et al. and Gebbia et al. studies, respectively), despite the fact that more than 65% of patients had already received 2 or more lines of treatment. In the Neuzillet et al. trial there is also great heterogeneity in the results despite the similar median overall survival of 6 months: the range of overall survival is 0.5-36.8 months vs. 2-8.2 months in the Gebbia et al. study, respectively. Finally, the Neuzillet et al. study does not state whether results or toxicity differed between patients receiving FOLFIRI-1 or FOLFIRI-3 regimens.
In conclusion, little progress has been made in the field of second-line treatment of gemcitabine-refractory pancreatic cancer; therefore, there is no evidence-based treatment recommendation for these patients. There is need for larger randomized trials that will study novel agents as well as new treatment combinations in an effort to improve survival while maintaining quality of life.
None