Research Article - (2015) Volume 23, Issue 2
National University of La Plata, Argentina
CONICET
Paula Risso
National University of La Plata, Argentina CIC, Buenos Aires, Argentina
Daniela Sbatella
National University of La Plata, Argentina
Griselda Haag
National University of La Plata, Argentina
Objectives: We examined the impact of personalizing antidiabetic agents in treatment adherence and in health outcome of socioeconomic vulnerable patients.
Methods: We conducted a randomized controlled trial comparing usual “on demand” drug supply from Primary Health Care (PHC) Centers; with a drug dispensing strategy based on a personalized process. 469 patients with type-2 diabetes (DBT) were assigned to either control (CG) or intervention group (IG) assuring a balance in main risk factors. Primary trial endpoint was treatment compliance; however aspects like health events associated to DBT were also evaluated.
Results: Adherence to oral agents in CG was 15.57% and 92.09% in IG (p <0.001). Dose omissions represented the most prevalent form of non-adherence. Weight loss was greater in the intervention group than in the control group throughout the study (5.4% in overall weight reduction at study end). Hospital admissions (16.80% vs 10.23% - p <0.01) and coronary heart events (7.37% vs. 3.72% p 0.03) were higher for CG when compared with IG.
Conclusions: Diabetes faces the challenge of lifelong treatment. This task is difficult for every patient but is especially hard in social vulnerable situation. Our initiative demonstrate that for the poor and uninsured population, personalizing drug dispensing showed a high level of treatment compliance, and benefits in terms of health consequences associated to diabetes. This experience might be useful for primary care health teams in order to better control social underserved patients, and also for reducing the economical impact for health system caused by complications due to untreated diabetes
Diabetes, Personalized, Treatment, Adherence, Primary Health Care
Diabetes (DBT) is a chronic disease present all over the world. In 2014, 9% of adults had diabetes. Over 1.5 million of annual deaths in the world are directly due to diabetes.[1] In addition, another 3.8 million deaths are attributable to indirect consequences of diabetes.[2,3] Noteworthy, more than 80% of diabetes deaths occur in low- and middle-income countries.[3]
Diabetes morbidity is related to an increased risk of heart disease and stroke, kidney failure, neuropathy (foot ulcers, and limb amputation), or retinopathy that cause blindness.[1,4]
The annual cost of type II diabetes per patient is $9,677.[5] The components of medical expenditures are related to hospital inpatient care (43%), medications to treat complications of diabetes (18%), physician office visits (9%), nursing facility stays (8%) and anti-diabetic type II agents (6.9%). Indirect costs are associated to absenteeism, job productivity reduction, inability to work, disabilities, and early mortality.[6]
As we can see, type II diabetes treatment has a low economical burden compared with taking care of the consequences of an uncontrolled disease.
In many countries, medicines for diabetes treatment are included in health programs. In Argentina, State health program provide free drugs and devices for any patient affected by diabetes disease. However, our group previously demonstrated that even if these programs provide free of charge treatments, patient’s compliance is low.[7]
Patients’ self care are negative influenced by the absence of symptoms of this disease. For this reason, patients are not aware enough of risks of leaving the disease to its own devices, and about the importance that entails ensuring treatment compliance.
Hence, it is clear that uncontrolled diabetes has severe health and economic consequences that may be avoid providing continuity to the treatment of this illness.
To demonstrate the impact of personalized drug dispensation in treatment adherence; we started an intervention study based on regular contact patients-health staff. This group was compared diabetic patients in whom drugs were provided “on demand” by standard ways of dispensing.
Patient population: social vulnerable adult patients diagnosed of type 2 diabetes, users of primary care health system from La Plata, Argentina were explored in order to check fulfillment of inclusion criteria. These criteria were age (>18 ≤ 65 years old), duration of diabetes (>2 <10 years), treatment (only oral treatment with either metformine or Glibenclamide-Glyburide was admitted) and social vulnerable status according to NBI index.8 Exclusion criteria were age >65 years old, motor disabilities, cognitive deficits, blindness, renal failure (clearance <30 ml/min) and hospitalization (for causes associated to DBT) or cardiovascular events (myocardial infarction, angina pectori, stroke, or heart failure) in the preceding year of the study.
Sample size: The sample size was estimated at 180 patients per study arm as a minimum to achieve a power of 80% at a 5% significance level for detecting a 0.2 absolute difference in the presence treatment compliance and any diabetes complications, between the intervention and control group.
Groups of Study: After we identified potentially eligible patients from the medical record system and drug provision list, we proceed to a random stratify selection of patients in two groups (Control and Intervention groups). Randomization process was performed using a table of random number. Patients included in the project received an information package, information about the study, and an informed consent form. Patients belonging to Control group (GC) were observed in the usual medical care process by their primary healthcare (PHC) provider or their own GP. Treatments were carried out with free drugs obtained on request either in the PHC service (REMEDIAR Program). On the other hand, Intervention group (IG) was submitted to the intervention strategy designed for this study.
Intervention: The intervention strategy consisted in assign to each patient, a person “responsible” for treatment assurance and drug dispensing. This person was an advance medical student. Each student had a maximum of 10 patients. A medical doctor or university professor was in charge of monitoring the care process of 10 students. Students had to contact “their” patients at least once a week, either in a personal visit or by phone contact. They had to assure that patients had enough available drugs for weekly treatment, and that patients had a daily compliance. If not, they could either establish a dispensing from the Primary Health Care Center or through home visit along with the pharmacist from the local primary care health service. All drugs box were personalized with name, surname and month of treatment for all IG patients.
Period of study: Between 1st January 2014 to 31st December 2014.
Variables of the study: age, gender, weight, height, BMI, drug treatment, treatment adherence -measure by MAQ survey, access to health service, number of medical consultation, hospital admission, inpatient days, creatinine clearance, smoking status, diastolic and systolic blood pressure, fasting glucose, HbA1c, urea, triglycerides and total, HDL and LDL cholesterol blood levels, neuronal, renal or cardiovascular events that demand consultation or hospital admission were also registered; social and economical status -measured by NBI index, parental history of diabetes, and cardiovascular risk status -measure by UKPDS risk engine and Framingham score.8-11 Al variables were measured before and after the experience in both CG and IG.
Endpoints: Primary end point was treatment adherence; and Secondary end points were complications associated to diabetes disease.
Statistical analysis: We used descriptive statistics to summarize personal and clinical data. The impact of the intervention was tested in the continuity of treatment and in reduction of complication associate to DBT. We present regression coefficients, indicating the mean difference between the intervention and control group after adjusting for baseline variables.
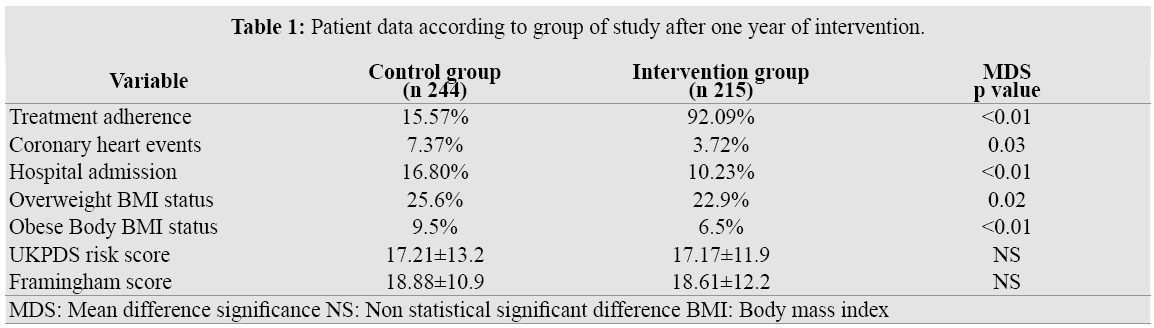
Four hundred and sixty nine patients were enrolled in the study. Two hundred and forty four were assigned to Control Group (CG) and 215 were included in the Intervention Group (IG). General baseline data for both groups demonstrated non significant differences in the main variables to be study (Table 1). Baseline systolic blood pressure level was 144, 11±20, 24 and 147, 42± 25,31 and diastolic blood pressure was 87,42±7,96 and 89,15 ± 9,25 either in control or intervention groups.
Twenty-two (22.0%) percent of subjects were taking two oral diabetic agents (metformine+glibenclamide) (CG 22.3% - IG 21.6%).
Cholesterolemia baseline level for control and intervention group, measured in mg/dl was 224,32 ± 45,85 and 225,46±49,34 respectively; while triglycerides blood levels were 231,32±166,24 mg/dl for the control group patients and 237,15±171,10 mg/dl for study group.
Non-significant differences were between both groups in relation to age, gender, smoking status, dyslipidemias, BMI or cardiovascular risk (measure by two different scores UKPDS and Framighan scores).
Hence, at the beginning of this study, we can assert that randomization process was successful since both CG and IG groups were initially balanced in main variables to be considered as outcome indicators.
After one year after that the study began, no significant differences were observed either in cardiovascular score risks (UKPDS: CG17.21±13.2; IG 17.17±11.9.or Framingham: CG18.88±10.9; IG 18.61±12.2 scores), dyslipidemias (CG: 28.2; IG 27.9%), or smoking status (CG23,6%; IG22.95). However, a small difference was register in coronary heart events. In CG 18 patients (7.37%) were admitted for heart problems while only 8 patients (3.72%) do so in IG (p 0.03).
Hospital admission for any cause related to diabetes disease was higher in CG than IG (16.80% vs 10.23% - p <0.01)
We noted in patients belonging to the intervention group, changes in BMI status (less obese and overweight patients) not only with regard to control group (BMI for obese category CG9.2%-IG6.5% (p< 0.01); and for overweight CG25.6%- IG22.9%) (p 0.02); but also to IG own baseline (10.31%/27.46% baseline compared to 6.5%/22.9% for obese/overweight respectively p<0.01 for both values).
IG HbA1c blood levels and hypertension were also improved but with less statistical significance when compared with either baseline (7.1 vs. 6.6 p 0.04 and 38.4% vs. 34.7% p0.03) or with control group after the experience (CG 6.9 vs. IG 6.8 p 0.04 and CG 36.7% vs. IG 34.7% p0.04). No differences were seen in renal, ophthalmological or neuronal events during the year of study.
Among control group patients receiving their medication from PHC centers, adherence to oral agents was only 15.57% (even less if receiving two drugs 10.65%). After twelve month of treatment, 92.09% IG patients were still taking regularly the prescribed oral medication (73.02% if treated with two drugs) (p <0.001). Dose omissions represented the most prevalent form of non-adherence; while overdose or dropout was only seen in 8.9/15.3% respectively for CG and 2, 7/4.3% for IG. Hypoglycemia was the main side effect seen in patients treat with glibenclamide (1.22 and 0.93 for CG and IG respectively).
Diabetes is responsible of severe consequences either to patients affected by this disease and also to the health system. Prevalence of diabetes mellitus type 2 has risen steadily over the past few decades all over the world. Patients attempted with this disease increased their risk of developing micro vascular complications like retinopathy, nephropathy and neuropathy, which, if untreated or with treatment dropout, can have a devastating impact on quality of life and place a significant burden on health care costs.
To reduce diabetes associated morbidity and mortality it is essential to guarantee controls and treatment compliance. However, since this disease have no symptoms; patients are less alert to fulfill the medical prescription.
In our present work we demonstrated that a personalized drug delivery may reduce treatment dropouts and helps to diabetes therapeutic compliance.
This treatment adherence demonstrated in our study that was able to reduce some of the disease consequences. [12]
All patients with type II diabetes faces the challenge of lifelong adhering to prescribed medications. However, if this task is by itself difficult for everyone, it is even harder to comply for social vulnerable people. Our initiative demonstrate that for the poor and uninsured population, personalizing drug dispensing showed a high level of treatment compliance, and so far, at only 12 months of intervention’s follow up, the harmful health consequences associated to diabetes like myocardial infarction, as well as hospital admissions were reduced when compared with control group. This experience might be useful for primary care health team in order to better control social underserved patients, and reducing the economical impact for health system caused by the complications medical attention associated to lack of diabetes treatment adherence.
A larger number of patients and a longer follow-up may demonstrate other benefits of this experience, so we will continue with present study in time.