- (2009) Volume 10, Issue 2
Christopher J Hoimes1, Alexios S Strimpakos2, Muhammad Wasif Sai
1Yale Cancer Center, Yale University School of Medicine. New Haven, CT, USA
2Department of Medicine, Royal Marsden Hospital. Surrey, United Kingdom
Pancreatic cancer is the fourth leading cause of cancer death in the United States and has a lower survival rate than other digestive tract tumors. It remains a therapeutic challenge with limited active agents. Honing our current understanding of markers of toxicity and response, and individualizing treatment with the prognostic and therapeutic tools available are important to make a worthy impact on a patient’s course. The authors summarize selected abstracts from the ASCO Gastrointestinal Cancers Symposium, San Francisco, CA, USA, January 15-17, 2009. The Symposium featured pancreatic cancer in 84 research abstracts, of which, seven are reviewed that focus on markers of toxicity: cytidine deaminase (Abstract #151) and haptogloin (Abstract #167) as markers of gemcitabine toxicity; markers of response: use of PET scan for prognosis (Abstract #157), and correlations with CA 19-9 to postchemo-radiation resectability (Abstract #215) and time to progression (Abstract #160); and individualized applications: characterizing the phenotypic similarities between a patient tumor and the direct xenograft (Abstract #154) and a report about the poor outcome of patients with ascites (Abstract #220). Validated clinical tools that can assist in managing patients through the narrow therapeutic window are needed.
Ascites; CA-19-9 Antigen; gemcitabine; Cytidine Deaminase; Haptoglobins; Pancreatic Neoplasms; Positron- Emission Tomography; Xenograft Model Antitumor Assays
CDA: cytidine deaminase; dCK: deoxycitidine kinase; FDG: fluorodeoxyglucose; mSUV: maximum standard uptake value; MTB: metabolic tumor burden
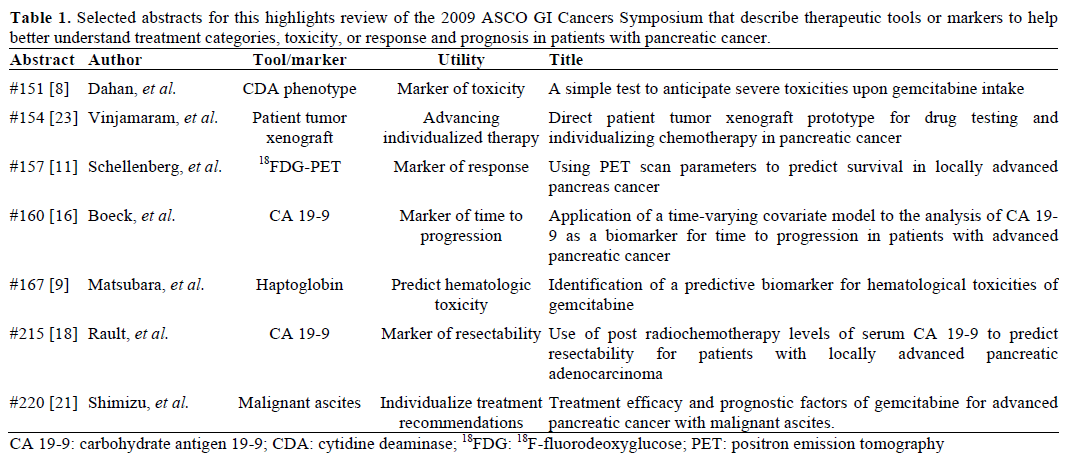
Pancreatic cancer is the fourth leading cause of cancer death in the United States [1] and remains a diagnostic and therapeutic challenge. The chances for achieving five-year survival are best with early diagnosis and treatment, though odds are still slim. Effective early detection and screening for average-risk populations are currently not available. Anatomically, the pancreas is different from other tubular parts of the gastrointestinal tract in that the retroperitoneal space is more difficult to access, sample, and image. Thus, most patients present with advanced disease at diagnosis with a median expected survival with best supportive care of 3-4 months. Symptom control is the focus of treatment and decision to recommend cytotoxic therapy is largely based on performance status. Existing clinical markers of pancreatic cancer lack specificity, as they are also found in inflammatory diseases of the pancreas and biliary tract. Better clinical tools that can assist in managing patients through the narrow therapeutic window are needed. The “2009 ASCO GI Cancers Symposium” featured pancreatic cancer in 84 research abstracts, of which we review seven that focus on markers of toxicity, response, and individualized therapy (Table 1).
Gemcitabine is a pyrimidine antimetabolite prodrug that requires cellular uptake. It is either inactivated by cytidine deaminase (CDA) to difluorodeoxyuridine (dFdU), otherwise it is activated by deoxycitidine kinase (dCK) where the nucleotide metabolite difluorodeoxycytidine 5’-triphosphate (dFdCTP) is incorporated into DNA resulting in chain termination. dCK is the rate-limiting enzyme for the activation pathway, and a patient’s phenotypic expression may play an important role in response to gemcitabine therapy [2, 3]. Similarly, CDA expression is instrumental for inactivation and may play an important role in gemcitabine toxicity.
The hypothesis that there is an association between tumor dCK expression and outcome has been evaluated in clinical trials of pancreatic cancer patients. Sebastiani and colleagues showed dCK protein expression from human pancreatic adenocarcinomas varied in immunohistochemistry labeling intensity [4]. They found that low intensity staining of dCK correlated significantly with both overall survival and progression-free survival when these patients were treated with gemcitabine based therapy.
Similarly, CDA mutations and phenotype have been implicated in anticipating gemcitabine toxicity. Sugiyama et al reported on polymorphisms that could be associated with gemcitabine toxicity in their patients [5]. Others have suggested that rather than mutations, CDA phenotype is perhaps a better predictor of toxicity, and mutations can lead to false negatives [6, 7].
Dahan et al. in Abstract #151 [8] report on a phenotypic test of CDA activity towards anticipating those at risk for gemcitabine toxicity. They evaluated baseline CDA phenotypes of 130 patients receiving gemcitabine in retrospective fashion and found large variability in CDA levels with a mean of 3.6±2.8 U/mg. Contrary to Sugiyama et al. [5] and other reports on genotypic correlations with toxicity, Dahan et al. found a trend of lower CDA levels with increased toxicity (P values were not reported). They did not find mutations of the CDA gene to be prognostically relevant for toxicity.
Matsubara et al. (Abstract #167) [9] focused on finding a test that would predict hematologic toxicity in patients with pancreatic cancer receiving gemcitabine. They used a case-control approach to evaluate the plasma proteomes of 47 patients divided into two cohorts of those without hematologic toxicity (grade 0), and those with grade 3-4 neutropenia and/or grade 2-4 thrombocytopenia. Using quantitative mass spectrometry, they found 757, 1.2%out of 60,888 peptide peaks sampled, that were significant (P<0.001). Out of these, haptoglobin was the marker found to have the lowest P value, and they argue, most statistically significant. They used haptoglobin in addition to neutrophil and platelet counts, body surface area, in a forward stepwise goodness-of-fit model to develop a nomogram which they prospectively studied in two independent validation cohorts of patients with pancreatic cancer. They found that baseline absolute neutrophil count and serum haptoglobin levels were statistically significant (P=0.0003 and P=0.031, respectively) with odds ratios (OR) of 0.72 and 0.71, in predicting grade 3-4 neutropenia or grade 2-4 thrombocytopenia, respectively. The authors do not specifically include a range of haptoglobin or neutrophil values that they determine to correlate, nor do they comment on other scenarios or clinical findings in their patients that would account for altered haptoglobin levels (such as intravascular hemolysis, or fucosylated haptoglobin[10]).
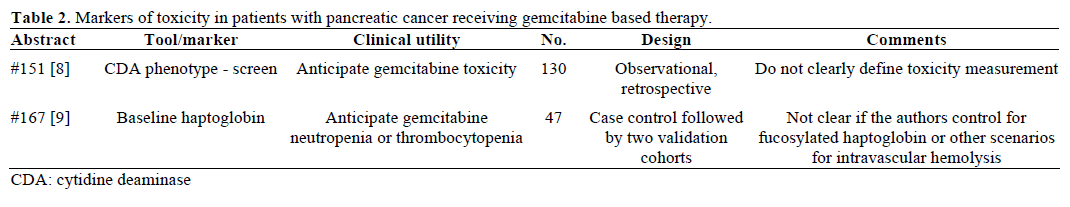
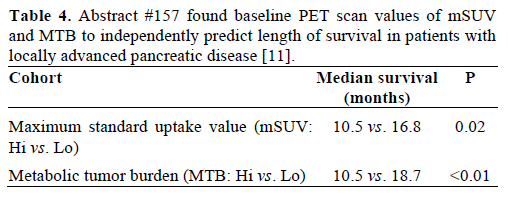
Positron emission tomography (PET) scan is not a routine part of staging for pancreatic cancer. There are situations where it may be obtained, such as postresection rising CA 19-9 with soft-tissue changes in the surgical bed seen on CT, or with equivocal or indeterminate findings in the liver or lung. The caveat is 18F-fluorodeoxyglucose (FDG) is often not detected in disease that is less than 1 cm. Schellenberg et al. (Abstract #157) [11] correlated baseline PET scan parameters with overall survival in locally advanced pancreatic cancer. They used open source software (developed at their institution and available for download at https://rtimage.sourceforge.net/index.html [12]) for measuring maximum standard uptake value (mSUV) and metabolic tumor burden (MTB) of baseline PET scans in 56 patients with locally advanced pancreatic cancer [13, 14]. Median survival was 12.7 months for all patients. They divided patients into two cohorts of high and low mSUV, and high and low MTB, and found significant differences between these groups (Table 4). When they further divided the patients into 4 subgroups of high and low mSUV and MTB, they found that the patients with both low MTB and mSUV had median survival of 18.7 months compared to the median survival of 9.3 months of the group that had high MTB and mSUV (P=0.058).
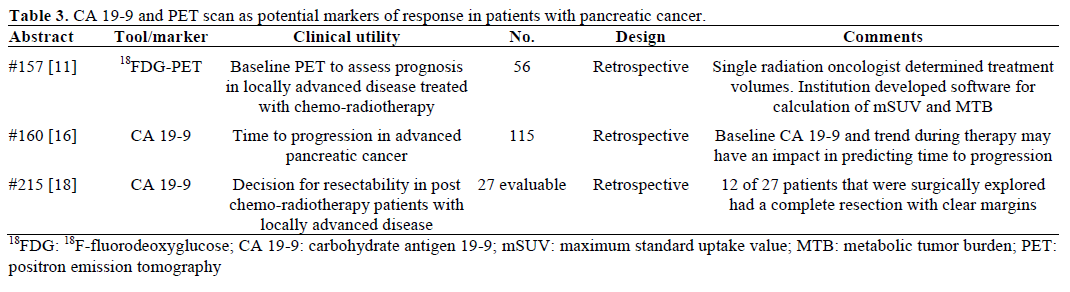
CA 19-9 is not specific for pancreatic cancer and may be elevated in other GI tumors, however it is the most commonly elevated serum tumor marker in pancreatic cancer [15]. The serum value corresponds to the CA 19-9 antibody response to the carbohydrate moiety of mucin 1 (MUC1). Decision for its use is based on mostly retrospective data, and it is still unclear if or how it should be used for prognosis, staging or preoperatively as an independent predictor of resectability. Boeck et al. (Abstract #160) [16] sought to analyze time to progression based on baseline versus trend of CA 19-9 in their multicenter retrospective analysis of non-resectable pancreatic cancer patients. They included 115 patients with confirmed pancreatic cancer and a pre-treatment CA 19-9 of at least 5.2 U/mL. Nearly 90% had metastatic disease, and most were treated on a clinical trial. Median time to progression was 4.4 months and univariate and multivariate analysis showed a significant (all P values <0.001) correlation of time to progression and pre-treatment CA 19-9 and CA 19-9 trend on treatment.
In 2003, Wolff et al. retrospectively examined pregemcitabine/ radiation levels of CA 19-9 in 79 resectable patients and found that patients with a CA 19-9 level of greater than 668 U/mL predicted radiographic presence of metastasis or early relapse [17]. Rault et al. (Abstract #215) [18] examined neoadjuvantly treated CA 19-9 values to predict resectability. Retrospectively, 33 patients with histologically confirmed pancreatic cancer were evaluated post-chemoradiation and 27 were surgically explored. Of this surgical cohort, there was a 2:1 ratio of patients with CA 19-9 less than 200 U/mL. Fifteen were able to have a pancreatic resection, for a more favorable resectability rate in patients with CA 19-9 less than 200 U/mL by a nearly 3:1 margin.
III. Individualized Therapy (Table 5)
Ascites in pancreatic cancer occurs in approximately 20% of patients and can be attributed to increased production of tumor exudates, osmotically active peptides that perturb vascular permeability, or obstruction of diaphragmatic lymphatics [19]. When it occurs ascites is usually the final manifestation, and it need not be proven to be malignant cytologically [20]. However, it is not clear if the amount of ascites matters. Shimizu et al. in Abstract #220 [21] performed a retrospective review of 80 patients with malignant ascites treated with gemcitabine, and looked for efficacy in cohorts of patients noted as having minimal, moderate, or massive ascites. Median survival was 4.2 months. Quality of life measures were not reported, though improvement in ascites was noted in 17.5% with a median time to treatment failure of 1.6 months. A multivariate analysis did show that amount of ascites, as well as performance status, were independent prognostic factors.
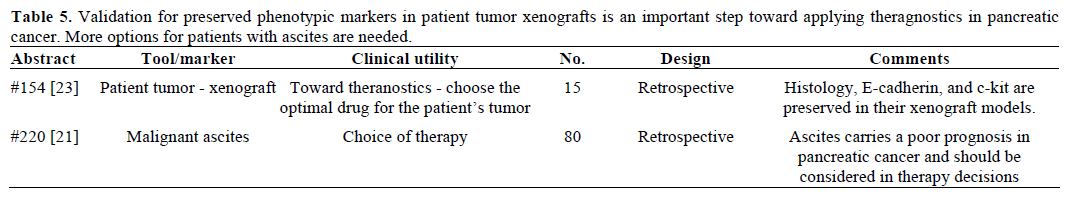
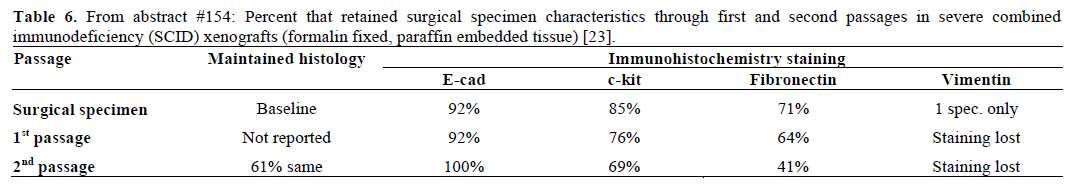
It is clear that more progress is needed in drug development for pancreatic cancer. Direct tumor xenografts can be used to test therapeutic approaches in pancreatic cancer; in fact, the model was recently used as a platform to advance targeting the pancreatic cancer stem cell [22]. An advantage is that all cellular fractions of the tumor can be evaluated; not just the tumor. However, it is not clear how well represented the patient’s tumor is in a mouse xenograft that has undergone some necessary processing in the lab, including passaging once or twice, for nude mouse implantation. Vinjamaram et al. (Abstract #154) [23] compared the histologic grade and immunohistochemistry staining of E-cadherin, c-kit, and other markers in the patient’s surgical specimen with that of first and second passages that were used as xenografts in severe combined immunodeficiency (SCID) mice. The specimens were formalin fixed and paraffin embedded for processing. They found that histologic grade was preserved between patient and the passaged cells. Staining for E-cadherin and c-kit was preserved through the passages, however, this was not true for fibronectin and vimentin (Table 6).
Better clinical markers can be beneficial for selecting patients for therapy, timing of therapy, and understanding prognosis of individual patients. With further study and validation, it is possible some of these tools make their way into randomized clinical trials and help advance therapy.
The authors have no potential conflicts of interest