Case Series - (2016) Volume 17, Issue 5
Mikram Jaffri, Amit H Sachdev*, Lauren Khanna, Frank G Gress
Columbia University College of Physicians and Surgeons, USA
Received Date: April 30th, 2016; Accepted Date: July 28th, 2016
Background Endoscopic ultrasound guided elastography is an imaging modality that can be used to evaluate tissue stiffness and to assess solid pancreatic lesions. It can also assist in optimizing the diagnostic yield of endoscopic ultrasound guided fine needle aspiration biopsies. Aims To review the literature on solid pancreatic lesions, the use of EUS guided fine needle aspiration and endoscopic ultrasound guided elastography and to present a single center experience using elastography to direct fine needle aspiration biopsies of solid pancreatic lesions. Methods We present a review of the literature and a single center experience describing the use of EUS guided elastography in directing fine needle aspiration biopsies of solid pancreatic lesions. Results Thirteen male veterans with an average age of 62.3 (SD±11.8) years were enrolled in the study. The mean pancreatic mass size on EUS was 5.1×5.2 (SD±4.4×4.5) cm. A total of 13 lesions were identified during elastography. The lesions were most commonly found in the body (n=5), followed by multifocal lesions (n=4), pancreatic head (n=3) and tail (n=1). The seven concerning pancreatic lesions were stratified based on color pattern identified on EUS and EUS-elastography. Three lesions were homogenously blue, and four lesions were heterogeneously blue. The remaining six lesions which were less concerning were predominantly green. Of the three lesions, that were homogenously blue, two were diagnosed as adenocarcinoma (n=2) and chronic pancreatitis (n=1) respectively. Of the four heterogeneously blue lesions two were adenocarcinomas, while the other two represented a large B-cell lymphoma and chronic pancreatitis. Patients whose lesions were characterized as homogenous or heterogeneous green were benign and remained disease free after a median of two years of regular follow up. Limitations Relatively small number of patients studied. Conclusions In our single center experience we found that the use of real time endoscopic ultrasound guided elastography for targeting fine needle aspiration of suspicious pancreatic lesions may be beneficial as an adjunct modality to complement conventional EUS. Larger prospective studies need to be conducted to evaluate the utility of this modality in targeting pancreatic lesions.
Biopsy, Fine-Needle; Elasticity Imaging Techniques; Endoscopic Ultrasound-Guided Fine Needle Aspiration
Traditionally, transabdominal ultrasound or computed tomography CT scanning [1, 2] are used to diagnose pancreatic lesions. However, transabdominal ultrasound is limited because it cannot be used to visualize the entire pancreas due to intervening fat or air. Endoscopic ultrasound (EUS) has emerged as the imaging modality of choice to diagnose chronic inflammatory, cystic, and neoplastic pancreatic lesions because it can assess areas of the pancreas at very close range [2, 3]. However, EUS is often times limited because it cannot distinguish between chronic pseudotumoral pancreatitis and pancreatic cancer. In some reports, the specificity of EUS images in diagnosing pancreatic masses or malignant lymph nodes has been described to be as low as 55% and the sensitivity of EUS-FNA is only 54% to 74% when sampling solid pancreatic masses in the setting of chronic pancreatitis [4, 5].
To help improve the diagnostic yield of endoscopic ultrasound, EUS guided elastography is increasingly being used in conjunction traditional endoscopic ultrasound. Elastography can be used to evaluate tissue stiffness and to assess the elasticity of solid lesions. Some studies have shown that EUS guided elastography is useful in improving diagnostic yield and efficiency of EUS-FNA of solid pancreatic lesions, however, this remains controversial [6, 7, 8, 9].
In this review and single center experience, we discuss what is currently known about pre-procedural and procedural techniques in EUS guided FNA biopsies of solid pancreatic lesions, and we evaluate the utility of EUS elastography in obtaining tissue samples for cytological and histological diagnosis. We also present our single center experience using EUS guided elastography to evaluate suspicious pancreatic lesions. We illustrate how the combination of EUS and elastography may help select the hardest area within a pancreatic lesion [10] and how it may be a useful adjunct for guiding further clinical management when EUS-FNAB is negative or inconclusive [11, 12].
When evaluating solid pancreatic lesions, one must understand that the differential diagnosis is broad and includes both benign and malignant lesions (Table 1) [13]. Of all solid pancreatic masses, primary pancreatic ductal adenocarcinoma is the most common accounting for over 85% of the lesions observed. Pancreatic adenocarcinoma is the fourth most common cause of cancer death in the United States [14, 15]. Most patients diagnosed with pancreatic adenocarcinoma are managed with chemotherapy, and as many as 20% undergo surgical resection. Tissue diagnosis is currently considered a prerequisite for chemotherapy and is desirable for those requiring surgery to exclude benign mimics of the disease.
EUS is the best modality to diagnose pancreatic tumors with sensitivity higher than 90%, especially for lesions less than 2-3 cm in size in which it reaches a sensitivity rate of 99% vs. 55% compared to CT [16]. EUS also has a very high negative predictive value, which is important clinically because it means that EUS can reliably exclude pancreatic cancer [17]. EUS is markedly superior to transabdominal ultrasound (reported sensitivity between 64%-91%) and has also been shown to be superior to computed tomography (CT) (sensitivity 66%-86%) for the detection of pancreatic masses in studies that compare both techniques [18, 19, 20]. However, often times it is difficult to differentiate between pancreatic cancer and mass-forming chronic pancreatitis, and EUS elastography has an important role in improving diagnostic accuracy in this subset of patients. In cases where a pathological diagnosis is required, EUS-FNA is particularly useful in guiding treatment decisions.
EUS FNA remains the gold standard in diagnosing pancreatic lesions and pancreatic cancer [21, 22] via tissue acquisition with a sensitivity of 80-85% and a specificity of 100% [23] however the diagnostic accuracy of EUS guided FNA is limited and EUS FNA can be associated with risks and complications [24]. There are many pre-procedural and procedural considerations that must be assessed prior to performing EUS guided FNA (Table 2). The location of the lesion, lack of adequate visualization, lack of experience of the endoscopist, lack of onsite pathology, and lack of adequate sampling are all limitations in diagnostic yield. False negatives can also occur in up to 40% of cases [21, 25].
When performing EUS FNA several considerations must be made [26, 27]. A careful history and physical examination must be performed. Patients usually present with jaundice and unexplained weight loss. The use of serum carbohydrate antigen 19-9 (CA19-9) level may be helpful in making the diagnosis by increasing the pretest probability of disease however it is important to note that a normal CA 19-9 does not exclude malignancy [28].
Patients who undergo FNA must have the appropriate indication for the procedure. In patients that have potentially resectable lesions, a FNA may not be necessary however it should be performed when considering neo-adjuvant therapy or when there is a concern for autoimmune pancreatitis in which management will be altered based on pathology results [14].
Prior to performing EUS FNA an evaluation of imaging is essential. Most pancreatic adenocarcinomas are hyopechoic. It is important to note that some lesions may not be well visualized or poorly defined on CT scan and this is more common in the setting of acute or chronic pancreatitis [29]. Cystic lesions, especially when complex or microcytic, may appear solid.
Adequate sedation, endoscopist experience [30, 31, 32]. Cytopathologist experience, technique used for tissue sampling, use of a stylet, number of passes, and molecular analysis also play a role in the diagnosis of pancreatic adenocarcinoma. Studies have shown that 25 gauge needles are easier to handle and are less likely contaminated with blood and may in fact have a better diagnostic yield than 22 gauge needles for cytological but not histological diagnosis [33]. In addition the method of tissue sampling also plays a role. The fanning technique, which involves sampling multiple areas within a lesion during each pass, was found to be superior to the standard approach because fewer passes were required to establish the diagnosis [34, 35, 36, 37, 38]. In our single center experience we focus on the use of EUS elastography and how it affects diagnostic yield. We used FNA when it was indicated, had appropriate imaging with either a CT scan or MRI prior to performing endoscopic ultrasound, had expert cytopathologists, had expert endoscopists, used 25 gauge needles, but did not use rapid on-site pathology (Table 3).

In our experience, no complications were observed, however, there are risks associated with EUS guided FNA. Risks of EUS-FNA include acute pancreatitis and needletrack seeding [39, 40, 41]. Contraindications to EUS-FNA include cases where patient management would not be affected, lesions, which cannot be clearly visualized, interposition of other structures, such as vessels, between the needle and the target and a risk of bleeding [11, 42]. In addition, false negative results can occur in the setting of chronic pancreatitis [25, 43]. Because the risks and contraindications and a need to improve diagnostic yield, a non-invasive imaging modality like EUS elastography may be beneficial.
EUS Elastography
Real time elastography performed during endoscopic ultrasound is a procedural technique that has been shown to better characterize pancreatic lesions during endoscopic ultrasound. Hiroka et al. first [44] reported the clinical usefulness of EUS elastography of the pancreas in 2005 and since then numerous reports of the utility of elastography has been published [45, 46]. There are two types of evaluation associated with elastography, a qualitative and a quantitative method. In the quantitative evaluation, image-analysis techniques are used to evaluate the characteristics of a lesion in a quantitative manner, including the use of a strain ratio, a strain histogram and a neural network. In the qualitative evaluation the pattern of the elastogram such as the major color tone and the heterogeneity of the color tones are examined.
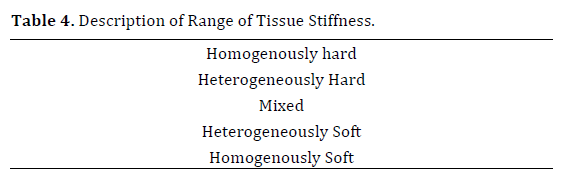
The quantitative evaluation of tissue stiffness can be divided into two techniques, strain and shear wave. In the strain technique, a compressive force is applied to tissue causing an axial tissue deformation (strain), which is then calculated by comparing the echo set before and after compression [47] (Table 4).
Longitudinal scanning and radial echoendoscopes can be used in elastography. Longitudinal echoendoscopes have the advantage that suspicious areas can be targeted for biopsy under direct visualization [48]. The area that is being examined is referred to as the region of interest (ROI). The ROI has to be sufficiently large, i.e. it must contain the area under evaluation and enough surrounding “normal” tissue for comparison. The optimum ratio is approximately 50% lesion, 50% normal/surrounding tissue. Elastography works by create a ratio of relative elastiscity difference between these two regions. If the ROI is small, this ratio cannot be displayed and elastography wont be useful [9, 49].
In the qualitative evaluation, the elastography software measures pixels of tissue in the region of interest and displays a hue of the relative strain value or hardness of tissue. Most of the systems display a chromatic map (redgreen- blue) in which hard tissue areas are shown in dark blue or blue and soft tissue areas are displayed in red or green. Because strain (deformation) is smaller in harder tissues and larger in softer tissues, the differences in strain (hardness) of the tissues can be quantified and by means of a color map displayed as a transparent overlay superimposed on the usual grey-scale EUS image [50] (Figure 1).
Figure 1: EUS and EUS elastography findings of four selected patients. (a). Elastography of a normal patient without pancreatic pathology, demonstrating a predominantly green pattern. This patient was not part of the trail and image is shown for comparison purposes. (b). Heterogeneously green pancreatic lesion, considered low risk. (c). Homogenously blue pancreatic lesion, diagnosed as adenocarcinoma on final pathology.(d). Heterogeneously blue pancreatic lesion, diagnosed as adenocarcinoma on final pathology.
The pancreas under EUS guidance has a smooth contour and is hypoechoic and finely granular. It appears homogenously soft compared to other tissue when evaluated under endoscopic ultrasound. With advancing age, pancreatic echogenicity increases significantly, because of fat and connective tissue deposits, and consequently the elastography image becomes more heterogeneous. The contour can become increasingly lobulated.
Studies have shown that endosonography when combined with elastography has the highest sensitivity for detecting even small pancreatic masses. In addition, some studies have shown that elastography can be beneficial in guiding biopsies for cytological and histological diagnosis [48]. The primary aim of EUS elastography is to distinguish benign and malignant tumors through the assessment of tissue elasticity (with benign tumors being soft while malignant tumors are hard) [51]. According to the seminal studies, benign masses are green and malignant masses are blue [52, 53]. Each color represents a different elasticity pattern and because there is a range, many qualitative analysis methods have been describe to best characterize the predominant color inside a region of interest. The most commonly used description has a range of: homogenously hard, heterogeneously hard, and mixed, heterogeneously soft and homogenously soft (Table 3).
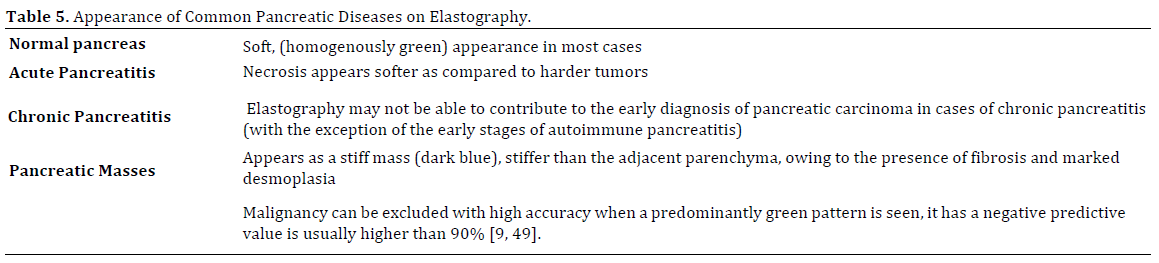
In clinical practice the normal pancreas has a soft (homogenously green appearance) in most cases. In acute pancreatitis, necrosis appears softer as compared to harder tumors. Chronic pseudotumoral pancreatitis can be differentiated from pancreatic adenocarcinoma by a difference in elastography appearance in most of cases, based on semi-quantitative analysis, which showed high strain for the average hue histograms in chronic pancreatitis as compared to pancreatic adenocarcinoma. However, often times chronic pancreatitis and hard tumors cannot be distinguished by elastography, probably because of their similar fibrous structure [54]. Pancreatic ductal adenocarcinoma appears as a stiff mass, stiffer than the adjacent parenchyma, owing to the presence of fibrosis and marked desmoplasia. Autoimmune pancreatitis has a characteristic stiff diffuse pattern of the whole pancreatic parenchyma, not just in the focal mass (Table 5).
It is important to note that studies have also shown that EUS elastography has been shown to be reproducible in the evaluation of solid pancreatic lesions, even between endoscopists with no or limited experience in EUS and/or EUS elastography. Reproducibility and diagnostic accuracy increase with experience in EUS and EUS-elastography [55].
Two large studies have evaluated the accuracy of strain based elastography and found the sensitivity ranging from 93% to 100% and the specificity ranging from 17% to 95% for diagnosing pancreatic malignancies [56, 57]. The specificity for diagnosing these malignancies is widely variable. In a large meta analysis of 13 studies with 1,042 patients with solid pancreatic masses elastography showed a pooled sensitivity and specificity of 95% (93%- 96%) and 69% (63%-75%), respectively [58].
A retrospective review of prospectively collected data collected at a large Veterans administration medical center from December 1st, 2009 to March 31st, 2012 was evaluated and the following data examined: patients personal data, diagnosis at admission, clinical history, conventional EUS examination, EUS-elatography, and final diagnosis.
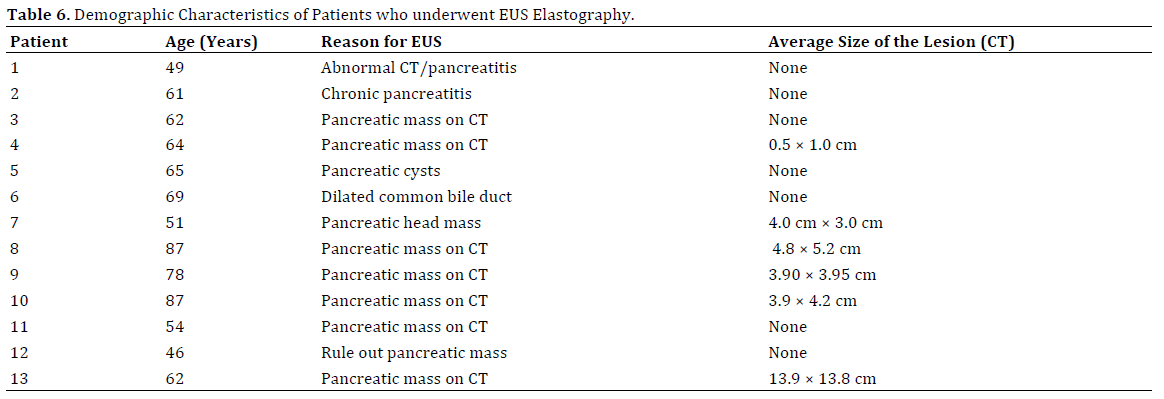
All patients above 18 years of age with prior imaging (Computed Tomography or Magnetic Resonance Imaging) demonstrating a pancreatic mass, or requiring further evaluation to better rule out a pancreatic mass, were included. Patients with pancreatic masses predominantly cystic in nature, with a history of previous pancreatic surgery or carcinoma, and patients who underwent EUS evaluation of extra-pancreatic disease such as peripancreatic lymph nodes were excluded (Table 6).
All endoscopic examinations were performed by two expert EUS endosonographers with at least one year of specialized training in endoscopic ultrasound. A qualitative and quantitative analysis was performed by each endosonographer. Each endosonographer analyzed elastography images based on the most predominant color and its distribution. Color patterns were classified as: 1) Predominantly green 2) Predominantly blue. Elastography patterns of distribution were classified as 1) relatively homogeneous pattern (homogeneous colors) or 2) heterogeneous pattern (areas of two or more different colors). EUS was performed using Hitachi HI VISION 900 or Preirus workstations (Hitachi Medical Systems Europe, Zug, Switzerland) with installed elastography module software using both linear and radial echoendoscopes (EG 3870 UTK, Pentax Orangeburg, NY). Pancreatic lesions were first examined using EUS and conventional B mode imaging (Hitachi HA 900) followed by EUS with real time elastography. EUS and EUS elastography were recorded during each procedure. For elastography, the probe was placed adjacent to the gastric or duodenal wall, exerting just enough pressure for an optimal and stable image on the standard B mode. The region of interest was identified and elastography was used to evaluate this region.
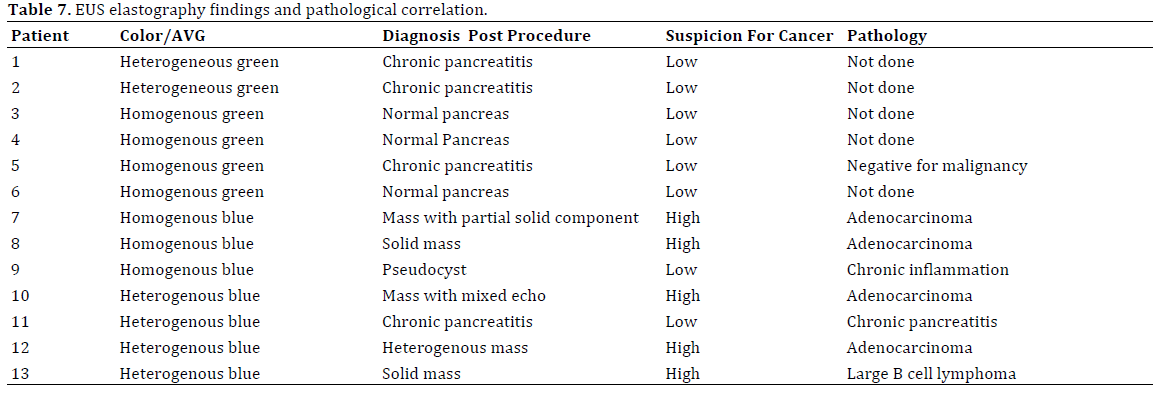
The mass was then classified as either homogenous or heterogeneous, blue or green, using criteria as previously described. The decision to perform EUSFNA of the pancreatic mass for cytology was based on the EUS appearance and the hue color pattern. EUS-FNA was targeted to a region of interest based on the color pattern (predominantly blue) or combination of EUS and elastography findings, while lower risk patients with a predominantly green pattern in lesions suggesting a benign lesion were followed clinically (Table 7).
A 22 or 25 gauge needle (Echo tip Cook Endoscopy Winston-Salem, NC) was used for sampling the mass. There was no on-site cytopathology service available, thus samples were collected and sent to the pathology lab to determine the final diagnosis.
Expert cytopathologists were blinded to endoscopic findings. The final diagnosis was determined by positive cytology or surgical pathology. Patients who were not biopsied (predominantly green pattern with low risk for malignancy) were followed clinically.
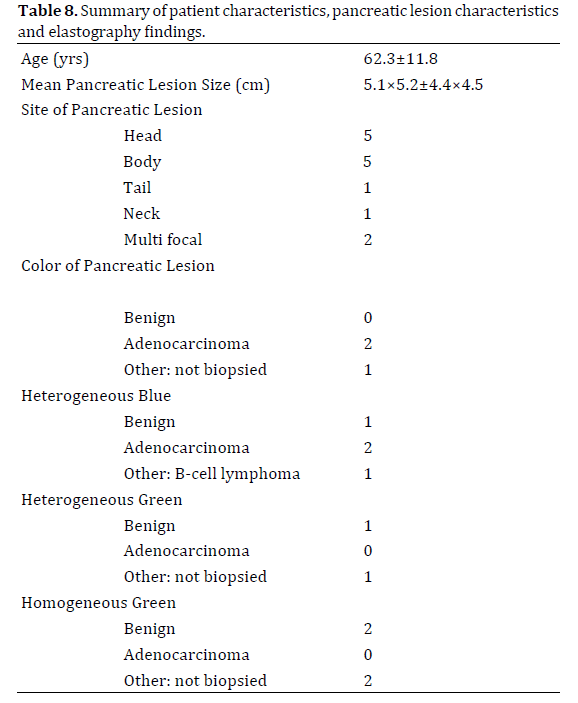
Thirteen male veteran patients with an average age of 62.3 (SD±11.8) years were enrolled in the study. All patients had abnormal findings on CT imaging prior to undergoing endoscopic ultrasound. The indications for EUS included CT findings of pancreatic mass/cyst, changes consistent with chronic pancreatitis and/or dilated CBD with clinical suspicion for malignancy.
The mean pancreatic mass size on EUS was 5.1×5.2 (SD±4.4×4.5) cm. A total of 13 lesions were identified during elastography. The lesions were most commonly found in the body (n=5), followed by multifocal lesions (n=4), pancreatic head (n=3) and tail (n=1). Of the 7 concerning pancreatic lesions identified on EUS and EUSelastography, three lesions were found to be homogenously blue, and four lesions were heterogeneously blue. The remaining six lesions were predominantly green. EUS-FNA was performed on six of the seven lesions that were blue on EUS-EG. Of the three lesions, that were homogenously blue, two were diagnosed as adenocarcinoma (n=2) and chronic pancreatitis (n=1) respectively. Of the four heterogeneously blue lesions two were adenocarcinomas, while the other two represented a large B-cell lymphoma and chronic pancreatitis (Table 2). One lesion characterized as homogeneous blue was not biopsied because of its location in the pancreas that would require too much of the pancreatic parenchyma to be traversed to reach the lesion.
There were six lesions that were characterized as predominantly green; 3 each as homogenous and heterogonous green. Of these, only one lesion characterized as homogeneously green was biopsied due to high clinical suspicion and was negative for malignancy. None of other predominant green lesions were biopsied (Table 8).
All patients including those who did not undergo biopsy based on low risk EUS and elastography findings were followed clinically for a mean period 2 years (range of 1.5-3 years). Follow-up included clinic visits with gastroenterology and primary care physicians. The patients underwent annual imaging or more frequently if clinically indicated. None of the patients that were not biopsied based on EUS and EUS-EG findings, nor those with negative EUS-FNA, developed an interval cancer, and all remained alive at the time of this completion of the study.
EUS elastography is a noninvasive technique that can be used to complement conventional EUS that requires minimal prolongation of the examination time, minimal cost, and no added risk of complications. Elastography can provide important information that may improve the diagnostic accuracy of solid pancreatic masses, especially when there are contraindications to FNA or when EUSFNA gives a negative result. In addition, elastography may help provide an optimal puncture site location when performing FNA and by doing so, may improve the diagnostic accuracy in a subset of challenging patients. Furthermore, elastography may reduce the number of false-negative cases and number of passes required to obtain adequate tissue for diagnosis.
Our single center experience demonstrates that EUS guided elastography may improve the diagnostic yield of EUS-FNA biopsies for pancreatic masses. Similar to previous studies, four elastographic patterns where defined quantitatively: homogeneous green pattern, heterogeneous green pattern, heterogeneous blue pattern, and homogeneous blue pattern. Using this classification, in our single center experience we identified pancreatic adenocarcinoma with a sensitivity of 100% and an accuracy of 85%. These results are comparable to previous studies which have shown that EUS elastography not only provides information complementary to that from EUS but also potentially increases the yield of fine needle aspiration and potentially reduces the number of unnecessary biopsies [59].
As with previous studies, differentiating pancreatic inflammatory masses from solid neoplastic lesions with similar rigidity was challenging. Inflammatory masses may produce a heterogeneous blue pattern, which parallels the color pattern of pancreatic neoplasms, as such, EUS elastography may not be able to target suspicious areas of a lesion or improve the low accuracy of EUS in patients with chronic pancreatitis. In our study, one of three patients diagnosed with chronic pancreatitis on prior CT had a mass confirmed on EUS elastography that was biopsied. The lesion biopsied was predominantly blue while the remaining lesions demonstrated a predominantly green pattern. EUS elastography has a high sensitivity but low specificity may provide false-positive results but not false-negative results for the diagnosis of solid pancreatic masses. Evaluation of the dominant hue in the color distribution on elastography is useful for assessing the diagnosis of pancreatic lesions but some degree of interobserver variation exists.
The strength of this observational study is its retrospective review of prospective date, participation of more than one expert endosonographer, and lack of an in-room cytopathologist, thereby providing unbiased verification of the reproducibility of the diagnostic utility of this technique in an independent cohort. An additional strength was the close follow up for two years for patients with a benign pattern on elastography. In our study, patients whose lesions were characterized as homogenous or heterogeneous green were benign and remained disease free after two years of regular follow up visits.
The limitations of our observational study included the small sample size. In addition, quantitative modifications in elastography like strain ratio, that were reported after our study was initiated, were not integrated into our study.
Although we report on the utility of EUSelastography to distinguish between malignant and benign pancreatic lesions, we cannot definitively conclude that elastography adds substantial value to EUS based assessment of solid pancreatic lesions because of our small sample size. Based on our experience we feel that elastography may be beneficial as an adjunct modality to complement conventional EUS. Given that larger, well conducted studies and meta-analysis have shown that EUS-elastography may or may not add substantial value to the EUS-based assessment of solid pancreatic lesions when compared to B-mode imaging with varying degrees of sensitivity and specificity [60]. We conclude that further studies must be conducted to evaluate the utility of EUS guided elastography.
EUS elastography may be helpful in characterizing pancreatic lesions and targeting EUS FNA biopsies. Furthermore, it can provide important information in differentiating pancreatic masses in cases where a negative result of EUS-FNA is suspected or in patients who are not suitable for FNA. We believe that this technique is likely to supplement rather than replace the role of pancreatic tissue sampling in the future. Further, well-designed large prospective studies need to be conducted to evaluate the utility of EUS elastography in directing fine needle aspiration biopsies of solid pancreatic lesions.
Authors declare no conflict of interests for this article.