- (2009) Volume 10, Issue 3
Carlos Alberto da Silva1, Karina M Cancelliero2, Dirceu Costa1,2
1Department of Physiotherapy, Methodist University of Piracicaba (UNIMEP). Piracicaba, Brazil.
2Department of Physiotherapy, Federal University of São Carlos (UFSCar). São Carlos, Brazil
Received November 19th, 2008 - Accepted March 17th, 2009
Objective The objective of the present study was to evaluate the effect of bleomycin sulfate on parameters related to the functionality of pancreatic tissue, with emphasis on the glucose tolerance test, insulin tolerance test, insulinemia and static secretion of insulin as well as the insulin receptor, and PKA, PKC and GLUT2 concentrations in the pancreatic islets. Design Twenty-four male rats were divided into 2 groups: control and treated with bleomycin (2.5 mg/kg, intratracheal mode). After 7 days, the animals were euthanized and the analyses were carried out. Statistics The normality and the homoscedasticity of the data distribution were tested and ANOVA was applied. The Tukey post hoc test followed ANOVA for the comparison of the static insulin secretion test at different glucose concentrations. Results In the glucose tolerance test, the bleomycin group showed a larger area (17,306±539 mg/dLx60min) than that of the control group (9,151±517 mg/dLx60min) and in the insulin tolerance test, there was a greater percentage fall in glycemia (8.08±0.56%) in the bleomycin than in the control group (3.87±1.14%). The bleomycin group also presented a reduction in insulin secretion and an increase in plasmatic insulin concentration in the static insulin secretion test. With respect to the concentrations of the insulin receptor, GLUT2, PKC and PKA in the pancreatic islets of the bleomycin group, there was an increase in GLUT2 (48.4%) and PKC (70.8%) and a reduction in PKA (38.5%). Conclusion During treatment with bleomycin, innumerable chemical-metabolic alterations were unleashed in the tissues which were not primary targets of the chemical therapy and which could compromise the homeostasis of the systems taking part in the glycemic adjustment, predisposing the organism to the development of a pre-diabetic pattern whose degree of incidence or reversibility is still unknown to the scientific community.
Bleomycin; Blood Glucose; Insulin; Pancreas; Pulmonary Fibrosis
SLC2A2: solute carrier family 2 (facilitated glucose transporter), member 2 (also known as GLUT2)
Pulmonary fibrosis is a respiratory disease which can be caused by the administration of bleomycin sulfate, an antineoplastic agent which can cause this disease as a side effect in the treatment of humans [1]. This toxic effect has been used in experimental models in animals [2], but the mechanism involved in the induction of the disease is still not completely understood [3]
It is important to point out that the time of administration of the bleomycin can influence the respiratory disease induced. According to Borzone et al. [4], the earlier stages of the pulmonary injury induced by bleomycin are associated with biochemical and functional changes which are similar to those of human pulmonary fibrosis whereas, in studies of more chronic stages, the pulmonary function changes are not compatible with a restrictive disease, but are more similar to those described in studies on humans with chronic obstructive pulmonary disease in which the mural inflammation and fibrosis of the bronchioles are associated with emphysematous changes.
There are various studies in the literature related to treatment with bleomycin, dealing with analyses related to the pulmonary tissue and the local and systemic effects resulting from the pathological condition of this tissue. However, presently, no study has been found concerning the effect of bleomycin on the pancreas, an organ of extreme importance to the organism, which could be influenced by treatment with this chemical therapeutic agent since it can cause alterations in tissue DNA due to its antineoplastic effect, and without tissue specificity, could cause pancreatic injury.
Thus, in the knowledge that treatment with bleomycin is widely used, and has already been described in experimental models with animals focused on the pulmonary fibrosis condition, the objective of the present study was to evaluate the effect of bleomycinsulfate on parameters related to the functionality of pancreatic tissue, with emphasis on the glucose tolerance test, insulin tolerance test, insulinemia and static secretion of insulin as well as the insulin concentration and the concentrations of protein kinase A (PKA), protein kinase C (PKC) and SLC2A2 [solute carrier family 2 (facilitated glucose transporter), member 2; also known as GLUT2] in the pancreatic islets.
The importance of this work in the evaluation of animal models should be emphasized, due to invasive analyses and the need for tissue biopsies, respecting the norms of the ethics committee on animal experimentation.
Animals
Twenty-four male Wistar rats (3 to 4 months old; 250- 300 g) were maintained under controlled vivarium conditions, with free access to food and water. The animals were divided into 2 groups (12 in each group): control and treated with bleomycin.
The treatment was carried out using a single dose of bleomycin (Oncoprod, São Paulo, Brazil; 2.5 mg/kg weight, intratracheal mode) [5] and, after a seven-day period, the animals were euthanized and the analyses were carried out.
Sampling
After the experimental period, the animals were sacrificed. The blood was collected and centrifuged, and the plasma was separated and dispatched for analysis. The pancreatic tissue was isolated and dispatched for evaluation. The animals of each group were divided into two subgroups (6 eah): one subgroup for the analysis of the static secretion of insulin and the other subgroups for Western Blot analysis (Sigma Chemical Co., St. Louis, MO, U.S.A.).
Glucose Tolerance Test
For the glucose tolerance test, the animals were anaesthetized with sodium pentobarbital (40 mg/kg weight, i.p.) and, after 40 minutes, an incision was made close to the femoral vein from where the blood sample was collected. After the first collection, glucose was injected (1 g/kg weight), new samples were collected after 5, 10, 15, 20, 30 and 60 minutes, and glycemia evaluated using a glucosimeter (Accu Check®, Roche, São Paulo, Brazil). The area under the blood glucose curve was evaluated.
Insulin Tolerance Test
Since the beta cell response to an overload of glucose was shown to be altered, an insulin tolerance test was carried out to evaluate tissue sensitivity. For the insulin tolerance test, the animals were anaesthetized with sodium pentobarbital (40 mg/kg weight, i.p.) and, after 40 minutes, an incision was made close to the femoral vein from where the blood sample was collected. After the first collection, 1 U/kg weight (1 U/mL) of regular insulin (Biobrás®, Montes Claros, Brazil) was injected; new samples were collected after 0, 2.5, 5, 10 and 20 minutes, and the glycemia was evaluated using a glucosimeter (Accu Check®, Roche, São Paulo, Brazil). Insulin tolerance test was represented by a percentage fall in glycemia (KiTT) in the presence of insulin.
Plasmatic Insulin
Plasma samples obtained by centrifuging the blood were sent for an analysis of insulinemia (ng/mL), carried out using a radioimmune assay (Du Pont New Research Products, Boston, MA, U.S.A.).
Static Secretion of Insulin
Islets of Langerhans were isolated using the technique described by Moskalewski [6] as applied to the murine pancreas by Lacy and Kostianovsky [7], with the modifications of Boschero et al. [8] and Sutton et al. [9]. After laparotomy and localization of the common bile duct, this was occluded at the extreme distal end close to the duodenum, and dissected close to the hepatic pedicle, where a polyethylene cannula was introduced in the discharge direction. About 8 mL of Hanks solution containing 8 mg of collagenase was injected via the cannula, causing rupture of the acinous tissue by retrograde flow. The pancreas was then ablated and transferred to a glass test tube (12x12 cm) and incubated for 18 minutes at 37ºC.
The contents, still at 37ºC, were then shaken vigorously for one minute and poured into a beaker. After mixing with Hanks solution, the contents were stirred slowly, ejected with a syringe and decanted for 3 minutes. The supernatant was discarded and the sediment resuspended in Hanks solution. After repeating this procedure 4 times, the final product was transferred to a Petri dish, from which, under a magnifying glass, the islets were collected by aspiration using a glass pipette with a long tapered tip. The isolated rat islets were collected in a polyethylene plate containing 24 wells, each containing 0.5 mL of Krebs-Ringer buffersolution supplemented with bovine albumin, to which glucose (5.6 mM) was added. All isolated pancreatic islets were collected and formed one pull of islets and each well contained 5 pancreatic islets. After incubating for 45 minutes (pre-incubation) at 37ºC in a carbogenic atmosphere (pH 7.4), the Krebs solution was substituted by 1.0 mL of the same buffer containing different glucose concentrations: 2.8, 5.6, 8.3, and 16.7 mmol/L. The plate was then incubated for an additional 90 minutes under the same conditions as above. After this period, the plates were placed in a freezer (-20ºC) for 10 minutes, and the supernatant in each well, separated from the cell, was transferred to polyethylene tubes and maintained at -20ºC until the secreted insulin was dosed one hour after the islets were collected. The normalization of the method was related to the use of the same number of pancreatic islets/well. During freezing, no evidence of cell breakage was found. The insulin secreted by the islets after incubation or plasmatic insulin was evaluated using the method described by Herbet [10], as modified by Scott et al. [11].
Dosage of the Insulin Receptor, PKA, PKC and GLUT2 in the Pancreatic Islets
Western Blot methodology was used to detect the cell expression levels of the insulin receptor, the extruded proteins, and the movement of the PKC and PKA insulin granules and of the GLUT2. Groups of 500 recently-isolated islets, incubated for 2.5 hours in Krebs solution containing 8.3 mM glucose, were centrifuged quickly and the supernatant discarded. Two-hundred μL of buffer specific for immunoprecipitation was then added and the islets were then polytenized in this solution for approximately 10 seconds, after which thhe homogenate was centrifuged at 3000 g for 10 min.
The precipitate was discarded and the protein dosed in the supernatant obtained, using the BioRad Protein Assay-Dye Reagent Concentrate (Melville, NY, U.S.A.). An albumin standard curve was used as the reference.
The samples were then incubated at 37ºC for 1 h in 20% by volume of Laemmli 5X buffer (0.1% bromphenol blue, 1 M sodium phosphate, 50% glycerol and 10% sodium dodecyl sulfate (SDS)).
The following biphasic gel was used in the electrophoretic run: stacking gel (4 mM EDTA, 2% SDS, 750 mM trizma base, pH 6.7) and running gel (94 mM EDTA, 2% SDS, 50 mM trizma base, pH 6.7). The run was carried out at 200 V for approximately 30 min with running buffer (200 mM trizma base, 1.52 M glycine, 7.18 mM EDTA and 0.4% SDS), diluted 1:4. The samples were transferred to a nitrocellulose membrane (BioRad, Melville, NY, U.S.A.). The transfer was carried out for 60 min at 30 V on ice, bathed by transfer buffer (25 mM trizma base, 192 M glycine).
After transfer, the membrane was blocked with 5% skimmed milk in tris saline solution (TBS) (1 M trizma base, 5 M NaCl, 0.5% tween 20) overnight at 4ºC. The proteins related to the study were detected on the nitrocellulose membrane by incubating at room temperature for 2 h, with the following specific monoclonal antibodies: anti-insulin receptor, anti-PKA, anti-PKC, and anti-GLUT2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A.; 1:500 dilution in TBS with 3% skimmed milk). The membrane was then incubated with the antibody conjugated with peroxidase HPB (1:5,000 dilution or 2 μg/mL in TBS buffer). The reaction with peroxidase was detected by autoradiography, soon after the reaction with Pierce’s Super Signal kit (Thermo Fisher Scientific, Rockford, IL, U.S.A.).
PCR was carried out on 12.5 μL of a mixture containing the following components: Taq polymerase buffer, 50 mM MgCl2, 10 mM of each deoxynucleoside triphosphate, 2.5 U/μL Taq DNA polymerase, 10 pmol of the primer sense, 10 pmol of the primer anti-sense and cDNA. Five μL aliquots of the PCR product were analyzed by gel electrophoresis in 1.0% agarose prepared in TBE buffer. After staining with ethidium bromide (0.5 μg/mL), the gel was photographed under UV light and quantified in an Eagle Eye II apparatus.
The animals were treated in accordance with the recommendations of the animal ethics committee which approved the project (protocol nº 754-2).
Data are reported as mean±SD. The data were initially tested for normality (Kolmogorov-Smirnov test) and homoscedasticity (Barlett criterion). Since the data presented normal distribution and homoscedasticity, analysis of variance (ANOVA) was used. The Tukey post hoc test followed ANOVA for comparison of the static insulin secretion test at glucose concentrations of 2.8 mM, 8.3 mM, and 22.2 mM, respectively. A twotailed significant level of 5% (P<0.05) was fixed. The software used was Origin 6.0® (Microcal Software, Inc., Northampton, MA, U.S.A.) and Prism® 3.0 (GraphPad Software, Inc., San Diego, CA, U.S.A.).
The bleomycin-treated group showed a statistically larger glucose tolerance test area (17,306±1,205 mg/dLx60min, P<0.001) than that of the control group (9,151±1,463 mg/dLx60min), as shown in Figure 1. The insulin tolerance test is shown in Figure 2. The treated group presented a greater percentage fall in glycemia (-8.08±1.36% P<0.001) as compared to the control group (-3.87±0.92%). This shows that bleomycin altered the insulin sensitivity since the speed of reduction of the glycemia was greater. Since there was an alteration in pancreatic functionality as shown by the glucose tolerance test, the study moved in the direction of a specific evaluation of the pancreatic islets, carrying out a static insulin secretion test where a significant reduction in insulin secretion at the three glucose concentrations (2.8 mM, 8.3 mM, and 22.2 mM) was observed (representing -40.0%, -35.5% and -55.5%, respectively) when compared with the control group (P=0.036, P=0.017, P<0.001, respectively) (Figure 3).
With the focus still on the pancreatic islets of the group treated with bleomycin, the concentrations of the insulin receptor, GLUT2, PKC and PKA were determined, observing significant increases in GLUT2 (48.4%) and PKC (70.8%) and a significant reduction in PKA (38.5%), as shown in Table 1.
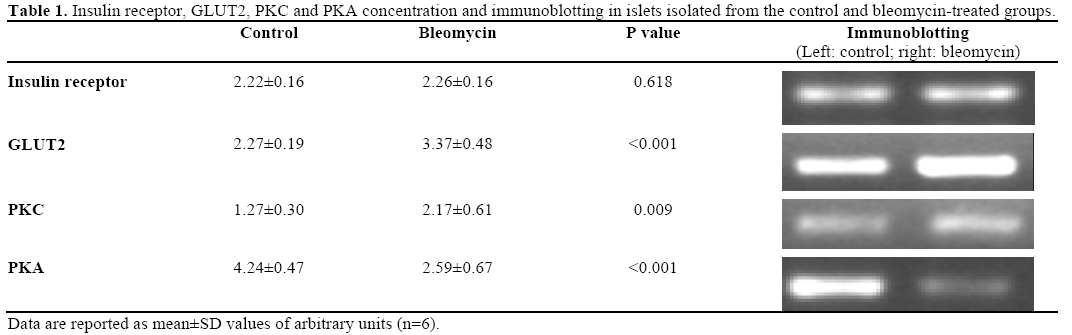
The plasmatic insulin concentration was also evaluated, showing a significant increase (P<0.001) in the treated group (7.81±1.71 ng/mL) as compared to the control group (0.83±0.19 ng/mL).
Insulin is undoubtedly an extremely important hormone in the regulation of glycemia homeostasis, and, thus, any physiological alteration of the endocrine pancreas reflects directly on the equilibrium of the synthesis/degradation ratio in the principal energy reserves [12].
Innumerable evaluation methods have been developed with the aim of determining the responsiveness of beta pancreatic cells, amongst which the glucose tolerance test stands out [13]. The action mechanisms of antineoplastic agents have long been a target of study and a guiding pivot of research studies [14]. In this context, the antineoplastic agent bleomycin deserves special attention since, even at low concentrations, this substance can induce oxidative stress and DNA alterations, particularly those related to the nitrogenized base thymine, expressively altering the cell cycles, a condition which stabilizes and/or inhibits the growth of neoplastic cells [15, 16].
In the present study, the glucose tolerance test showed that treatment with bleomycin induced supraunevenness of the area, indicating that the sensitivity of the beta pancreatic cells had been compromised. In an attempt to explain this, it is important to reflect on the changes in the secreting behavior of the insulin. In this context, the study was initially focused on the fact that the bleomycin expressed its action on the DNA, indicating that it was a highly fat soluble substance, with access to the nuclei of different cells and which possibly presented a greater intensity of action in cells with high metabolic activity, such as tumour cells. However, one should not ignore the possibility of its action in non-cancer cells whose homeostasis could also be affected by bleomycin.
In the context of this discussion, it is well known that amongst the mechanisms related to the action of the antineoplastic substance bleomycin, great relevance has been attributed to its capacity of promoting the formation of free radicals [17]
It is known that beta pancreatic cells possess high metabolic activity, regulated in a multi-factorial way especially when faced with an increase in extra-cellular glucose concentration, adenyl cyclase activators or phospholipase C activators (acetyl choline and cholecystokinin) [18, 19].
With respect to the action of bleomycin on the insulin secretion process, it was observed that islets isolated from rats treated with bleomycin presented an increase in the concentration of GLUT2, a transporter which expresses itself with high intensity in beta cells, indicating that, when faced with an overload of glucose, it would rapidly reach the Km of GLUT2, making the capture of large amounts of glucose possible, a fact which predisposes the organism to an increase in glucolytic flow, change in the ATP/ADP ratio, change in the cytosolic calcium concentration and consequent increase in insulin secretion, associated events which could induce an increase in the generation of reactive oxygen species [20]. In this way, concomitant with the increase in the cytosolic glucose content in the beta cells, there is also an increase in free radical formation and changes in the responsiveness of the insulin secretion process represented by the larger area under the curve after a glucose overload in agreement with various studies which suggest these changes [21, 22]. In this way, bleomycin can reduce the efficiency of the secretion process by the beta cells and possibly unleash a pre-diabetic status.
The oxidative stress generated by treatment with bleomycin is also one of the factors contributing to injury of the beta cells of the pancreatic islets which, on presenting increased activity of the AMP-activated protein kinase (AMPK) system, start producing a large amount of reactive oxygen species which, on activating the caspase enzyme and the B-cell CLL/lymphoma 2 (BCL2) gene segment, become predisposed to cell apoptosis, compromising the responsiveness of the insulin secretion cells, reinforcing the supraunevenness of the glucose tolerance test curve described above even more [23, 24, 25, 26].
Of the insulin secretion-inducing mechanisms, the importance of the mitochondria as integrating agents in generating energy should be emphasized since this determines the efficiency of the secretion process, and the mutations and alterations in mitochondrial function inducing a considerable production of reactive oxygen species which could take part in injuring the beta cells as has already been identified in diabetes [27, 28]. Due to the alteration of the responsiveness of the beta cells as indicated by the glucose tolerance test, the next objective was to evaluate insulin secretion by islets isolated from control animals, and compare them with islets isolated from bleomycin-treated rats. A reduction in the secretory response was found even in the presence of secretagogue concentrations of glucose, such as 8.3 mM and 22.2 mM, and, thus, the results presented here are of extreme importance since they show that treatment with bleomycin compromises the insulin secretory process induced by glucose and could be a reflex of possible oxidative stress resulting from the toxicity of the substance in the beta cells or could even strengthen the glucotoxicity produced by an elevated hexose capture [29, 30].
Thus, a more judicious evaluation of the insulin secretory process was carried out, evaluating the concentrations of the C (PKC) and A (PKA) protein kinases. The data showed a lack of functional equilibrium due to a significant increase in the PKC concentration accompanied by a reduction in PKA enzymes which, in an integrated and synergic way, regulate the steps of the insulin secretory process [31]. It is worth pointing out that an increase in the GLUT2 population was observed in the pancreatic beta cells isolated from the bleomycin-treated rats, offering ideal conditions for an increase in the rate of glucose capture in this micro-environment, concomitant with an increase in the cytosol glucose concentration. In this context, it has been described that an increase in glucose concentration is a strong PKC activating stimulus and, thus, the present study accompanies and corroborates the suggestion of Ha et al. [32].
One important point to be made is that the action of bleomycin is possibly related to alterations in the efficiency of metabolic pathways since PKA acts on the speed of movement of the vesicles containing insulin granules, and PKC acts in the co-localization of the enzymes involved in the secretory process and of the insulin granules, aiding in the movement of the vesicles to the cell periphery [33]. Thus, transporting these observations to the target cells, that is, to the tumour cells, this suggests that bleomycin causes disorder in the synergism between the kinases and, due to this, causes a lack of equilibrium in the efficiency of the energy-generating processes which is essential in keeping the cell alive. It is interesting to point out that Lee et al. [34] showed that reactive oxygen species amplified the expression and action of PKC when one considers the behavior of PKC in the functionality of the pancreatic beta cell. The action of bleomycin as an agent which increases the formation of free radicals can indirectly compromise the secretory process and implant a pre-diabetic status, a result which was recently confirmed in the study of Pi et al. [35].
On the other hand, on examining plasmatic insulinemia, an increase was shown in the bleomycin treated group, a fact which led to the hypothesis that bleomycin could induce apoptosis in the pancreatic beta cells promoting hyperinsulinemia since, when an islet dies, it transfers its insulin content to the plasma, as has already beenreported in the initial phases of the development of diabetes mellitus. In this sense, one can expect to find the implantation of a diabetogenic status connected to the treatment.
Another evaluation carried out was the insulin tolerance test with the objective of evaluating the behavior of the peripheral tissues, using the proposal of Cobelli et al. [13]. The analysis of the index of the fall in glycemia showed that there was a significant increase in the rate of glucose capture by the peripheral tissues, indicating changes in tissue responsiveness. This data suggest that insulin resistance could develop with a constant stimulus and, in this sense, some considerations are important. Two hypotheses were initially raised, that is, faced with a reduction in insulin secretion induced by the bleomycin, there is an upregulation of the insulin receptor population in the peripheral tissues, causing hypersensitivity of the glucoregulatory system so as to compensate and provoke an increase in the hexose capture speed, justifying the results found in the insulin tolerance test. On the other hand, one cannot discard the hypothesis that the free radicals generated by treatment with bleomycin injured the energy-generating systems, and could, in a second phase, compromise the insulin sensitivity of the peripheral tissues. In this way, recent studies have demonstrated that, in the insulin resistance condition, a reduction in mitochondrial activity and deficiency in oxidative phosphorylation were observed as well as the induction of morphological modifications of the mitochondria [36, 37, 38, 39].
Thus, during treatment with bleomycin, innumerable chemical-metabolic alterations are unleashed in the tissues, which are not the primary targets of the chemical therapy, and which could compromise the homeostasis of the systems taking part in the glycemia adjustment, and predispose the organism to the development of a pre-diabetic pattern whose degree of incidence or reversibility are still unknown to the scientific community.
It is important to point out the limitations of this study, one of which being the dosage used, which, although according to the literature, is the sole limitation. On the other hand, the period of treatment characterized the induction phase of the restrictive pattern, and other periods preceding the chronic phase, should also be analyzed.
Research supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) (protocol 04/14798-5).
The authors have no potential conflicts of interest