Keywords
Clofibric acid; Chromium (III); IR; Potentiometry; UV-Vis; Speciation diagrams
Introduction
Peroxisome proliferator-activated receptors (PPAR)s constitute a family of receptors; members of the steriod receptor superfamily [1-4]. To date, there are three known receptors, namely PPAR (α), (β/δ), and (γ). Since their discovery, PPARs have been shown to be expressed in monocytes/macrophages, the heart, vascular smooth muscle cells, endothelial cells, and in atherosclerotic lesions. Since PPARs are nuclear transcription factors; they regulate multiple genes involved in energy production, glucose and lipid metabolism [4-8]. Polymorphisms in these receptors influence the pathology of numerous diseases including obesity, diabetes, atherosclerosis, inflammation and cancer [1-8]. Furthermore, PPARs can be activated by a vast number of compounds including synthetic drugs such as the clofibrate and anti-diabetic thiazoldinedione classes, polyunsaturated fatty acids, and a number of eicosanoids, including prostaglandins, lipoxygenase products, and oxidized low density lipoprotein [6,9].
Yokoyama et al. [7] have investigated the inhibitory effect of clofibric acid (CA), a ligand for PPARα, on growth of ovarian malignancy in vivo and in vitro experiments using OVCAR-3 and DISS cells derived from human ovarian cancer and aimed to elucidate the molecular mechanism of its antitumor effect. CA treatment significantly suppressed the growth of OVCAR-3 tumors xenotransplanted s.c. and significantly prolonged the survival of mice with malignant ascites derived from DISS cells as compared with control. The results in this study indicated that CA produced potent antitumor effects against ovarian cancer in conjunction with a reduction of angiogenesis and induction of apoptosis [7].
The detailed literature review conducted in the current study revealed that a very limited number of publications have appeared that dealt with the interaction of CA with metal ions, especially in aqueous solutions [10]. Moncol et al showed the crystal structures and the spectral properties (UV-Vis, EPR) for both the bis Cu(CA)2 complex and the dimeric (Cu)2:CA complexes [10]. Ghauch et al. studied the effect of CA on metallic Iron (Fe0) and other iron plated surfaces such as plated Fe0 (mFe0: Fe0/Pd0, Fe0/Ni0) [11]. This paper by Ghauch et al. was not biologically relevant because iron was in its metallic form and not in aqueous environment. Although there is a wealth of toxicological, biological, medicinal, and chemical information about CA and PPAR [10-66] yet, there is lack of chemical studies of CA with essential and toxic metal ions under ambient conditions.
We have initiated a series of studies of CA with a series of metal ions such as Fe3+, Cu2+, Zn2+, and Cr3+. Clearly, these metals are essential metal ions that are found in the biological sphere with various degrees of abundance [67]. The choice of Cr3+ to be presented in the current report was due to the fact that Cr3+ is considered to be an essential metal that forms what is known as “the low-molecular-mass chromium-binding complex” (LMMCr) [68], which plays a role glucose metabolism [69,70].
Fibrates are the only marketed PPARα agonists that are effective in lowering elevated serum triglycerides. Their chemical structures are characterized by the presence of the 2-phenoxy-2- methylpropanoic acid moiety. The main objectives of the current report are 1) To show whether CA binds with Cr3+ or not?; 2) To identify the type of metal-complexes formed, if there is binding, under the ambient conditions, and 3) To measure the potential response of the reaction mixture in milli-Volts. Potentiometry is one of the most powerful tools to study metal ions in aqueous solutions at ambient conditions [71-74].
Experimental Section
Materials
All solutions were prepared using 99% purity Sigma reagent grade CA, C10H11ClO3, formula weight 214.6 g.mol-1 and chromium nitrate nona-hydrate, Cr(NO3)3âÂâ€â€ÂÂÂÂÂ9H2O, formula weight 400.15 g.mol-1, using doubly deionized water to prepare all solutions. Scheme 1 shows the structural formula of CA, or [2-(4-chlorophenoxy)-2-methyl propanoic acid (Chemical formula C10H11ClO3). The pH values of all solutions were adjusted using ~ 0.1 mol.L-1 sodium hydroxide (NaOH), solution that was standardized to the fourth decimal place. The pH values were measured using Orion Membrane pH meter (model 720) with a combination Orion-glass electrode in 0.1 mole.L-1 ionic strength using the appropriate amounts of 1.0 M NaNO3 solution.
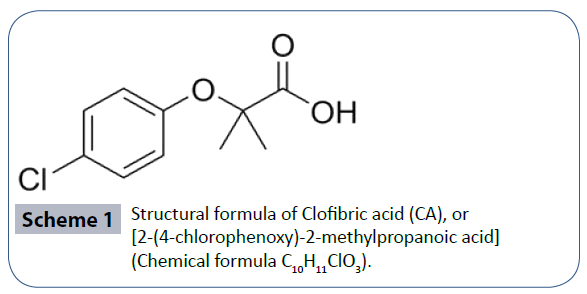
Scheme 1 Structural formula of Clofibric acid (CA), or [2-(4-chlorophenoxy)-2-methylpropanoic acid] (Chemical formula C10H11ClO3).
Preparation of the potentiometric titration solutions
In all metal-ligand potentiometric titrations, the NaOH solution was always the titrant. The NaOH solutions were prepared from NaOH laboratory grade pellets in carbonate free water. The methods used to prevent the contamination of the titrant with atmospheric CO2 had been described elsewhere [70-74]. The NaOH solutions were standardized using primary standard potassium hydrogen phthalate (KHP). Both NaOH and KHP were purchased from Fisher Chemical Co. Before any KHP titration, the KHP was dried at 110°C for 24 hours and stored in a desiccator. A stock indicator solution of about 0.2% phenolphthalein in about 90% ethanol was prepared from reagent grade phenolphthalein. KHP was titrated to the phenolphthalein end point. Typically, thirteen-fifteen runs were carried out to standardize the NaOH solution. Standard statistical treatments of the data such as the arithmetic mean, standard deviation, T-test, and Q-test were conducted using Excel software.
Potentiometric titrations
The potentiometric titration solutions were contained in a 250 mL beaker equipped with a magnetic stirring bar. The beaker was covered with a custom made Teflon cover. In a typical titration; the CA solution was added first followed by the addition of the Cr3+ metal ion solution. To adjust the ionic strength of the solution to 0.1 M the appropriate amount of 1.0 M NaNO3 was added. The total volume of the final titration solution was 100 mL. The final concentration of the metal ion titrated was in the range of 2.0 to 2.5 mmoles.L-1. Before each titration, the titration solution mixtures were allowed to stir for 25 minutes to reach equilibrium. The NaOH titrant was added in the 100 L increments using an Eppendorf micropipette with continuous stirring. The time intervals between the additions of the NaOH solution were set to 5 minutes, which was sufficient to get each of the pH values stabilized and reach complete equilibrium. The start pH-value was in the range of 3-4 and the final pH-value was in the range of 10-11. Each titration took about 5 to 6 hours to complete. All titrations were conducted at room temperature.
UV-Vis spectroscopy
All UV-Vis spectroscopy measurements were conducted using a T60 high-performance spectrophotometer in connection with UVWIN software version 5.0, both purchased from Advanced ChemTech (Louisville, KY). Samples were prepared in D.I. water at 25ºC. The entire UV-vis spectrum was scanned from 200 to 1100 nm using quartz cuvettes with optical path length of 1 cm. A reference cuvette filled with D.I. water was used with all measurements. The concentration of the metal was = 4.3 × 10−2 mol.L-1. The UV-Vis spectra were collected at the pH values of 3.00.
IR spectroscopy
All IR spectroscopy measurements were conducted using Nicolet iS10 spectrophotometer in connection with OMNIC software version 8.1, both purchased from Thermo Fisher Scientific (Madison, WI). Samples were prepared in D.I. water at 25ºC. The entire IR spectrum was scanned from 400 to 4000 cm-1 using the provided attenuated total reflectance (ATR) accessory cell compartment equipped with a diamond cell that can accommodate all kind of samples (solid samples or aqueous solution samples). The following data parameters were used in collecting the IR spectra: number of sample scans and the number of background scans was set at 32 with resolution of 4.000, and Laser frequency of 15798.7 cm-1. Typical IR spectra were generated in which the X-axis was given as Wavenumbers in cm-1 and Y- axis was recorded as % Transmittance.
Results and Discussion
Potentiometric titrations of free CA and free metal ions
Potentiometric titration experiments of free CA showed that the acidity constants of the carboxylic acid functional groups present to be pKa = 4.32 ± 0.06 at 25°C, 0.1 M NaNO3. To the best of the researcher’s knowledge, this is the first time to report the acidity constant of CA in aqueous solutions at room temperature in 0.1 M ionic strength. (Figure 1) is the potentiometric titration graph of free CA. This graph contains three overlapped plots to show data consistency. (Figure 2) is the speciation diagram of free CA generated in aqueous solutions using Hyperquad simulation and speciation (Hyss) software program [75]. pKw value of 13.78 was taken from the literature [76]. CA releases one proton due to the fact that CA has a sole titratable functional group; the carboxylic acid group. This confirms the fact that clofibric acid is a monoprotic acid. These data of this ligand has not been reported in the NIST standard reference database of critically selected stability constants of metal complexes [77].
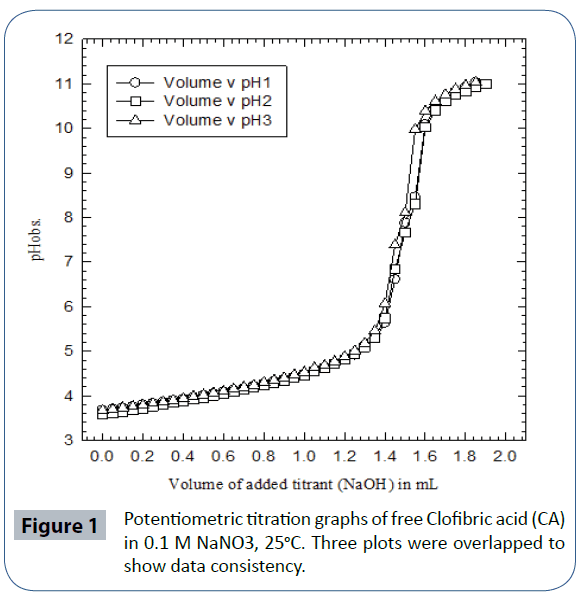
Figure 1 Potentiometric titration graphs of free Clofibric acid (CA) in 0.1 M NaNO3, 25°C. Three plots were overlapped to show data consistency.
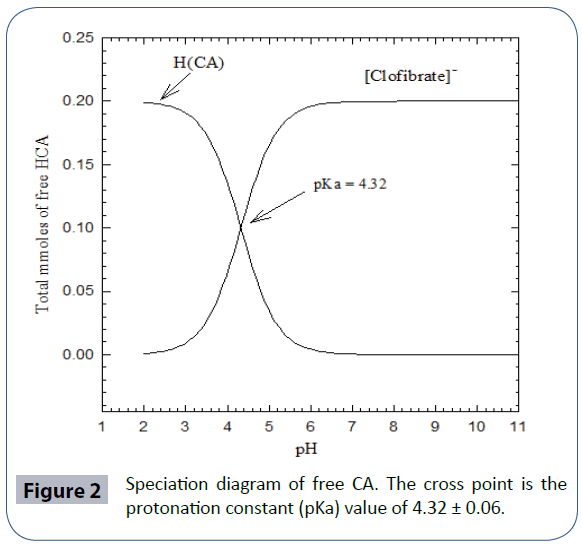
Figure 2 Speciation diagram of free CA. The cross point is the protonation constant (pKa) value of 4.32 ± 0.06.
(Figure 3a) is the potentiometric titration graph of free Cr3+. Four titrations plots were overlapped to show data consistency. (Figure 3b) is the mathematical treatments graphs for (Figure 3a). This mathematical treatment is the first derivatives (slopes) versus the number of observed equivalents. After data treatment and converting the volume of titrant into number of equivalents of titrant, it is clear that a tri-valnet metal ion such as Cr3+ releases a net of three proton equivalents into the aqueous solutions. This is due to the fact that metal ions in aqueous solutions under ambient conditions go through metal ion hydrolysis. This term, metal ion hydrolysis, is defined in equations 1-3 [78-80] and it is valid for any metal ion such as the chromium in its trivalent oxidation state. The number of equivalents is defined as the number of milli-moles of added titrant per number of milli-moles of Cr3+ ion present in solution.
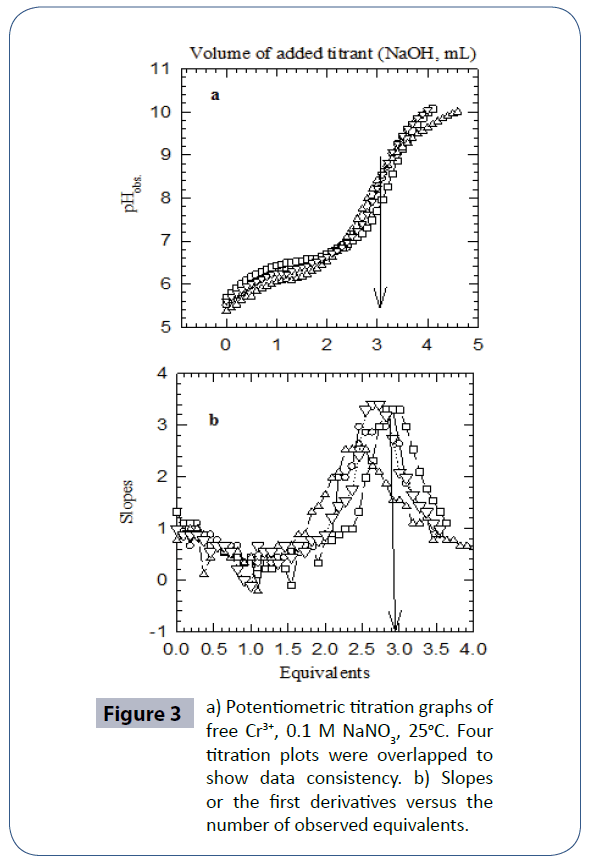
Figure 3 a) Potentiometric titration graphs of free Cr3+, 0.1 M NaNO3, 25°C. Four titration plots were overlapped to show data consistency. b) Slopes or the first derivatives versus the number of observed equivalents.
Cr (H2O)63+ → Cr (H2O)5(OH)2+ + H+ Eq. 1
Cr (H2O)5OH 2+ → Cr (H2O)4(OH)2+ + H+ Eq. 2
Cr (H2O)4(OH)2+ → Cr (H2O)3(OH)3 + H+ Eq. 3
Titrations of Cr3+ with CA
(Figure 4a) is the potentiometric titration graph of the Cr3+:CA in 1:1 molar ratio. This graph contains a total of three individual plots. This graph shows the exact locations of the inflection points. The location of each inflection point gives the exact number of protons released into the aqueous solution. For example, the titration plots of the Cr3+:CA in 1:1 molar ratio indicated the release of four protons. By examining these plots in this figure, clearly there has been a strong interaction between the metal ion Cr3+ and CA due to the shift in the location of the inflection points to 4.0 equivalents; compared to 3.0 equivalents in the titration of the free Cr3+ ion as shown in (Figure 3). (Figure 4b) is the mathematical treatments graphs for (Figure 4a). This mathematical treatment is the first derivatives (slopes) versus the number of observed equivalents.
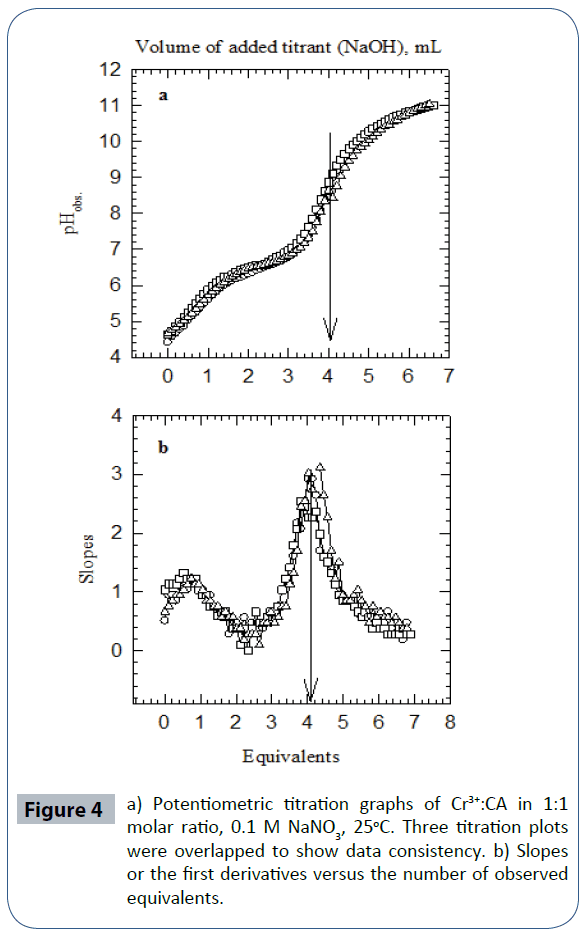
Figure 4 a) Potentiometric titration graphs of Cr3+:CA in 1:1 molar ratio, 0.1 M NaNO3, 25°C. Three titration plots were overlapped to show data consistency. b) Slopes or the first derivatives versus the number of observed equivalents.
For the Cr3+:CA in 1:1 ratio, the three replicas overlapped at 4.00 equivalents. The important point here is that four equivalents of protons have been released from the reaction of Cr3+ with CA into the solutions. One proton was clearly released from the CA. The source of the other three protons must be accounted for. These three protons have to come for the aqua ligand attached to the metal ion. It is established in the literature that such hydroxo-complexes with Cr3+ have been seen previously [72,79,80]. The proposed and the most plausible species to be formed in solution will be the ternary chromium hydroxo-clofibrate complex [Cr3+(clofibrate-)(OH-)3]1-. We are proposing the structure of this ternary chromium complex in aqueous solution in Scheme 2.
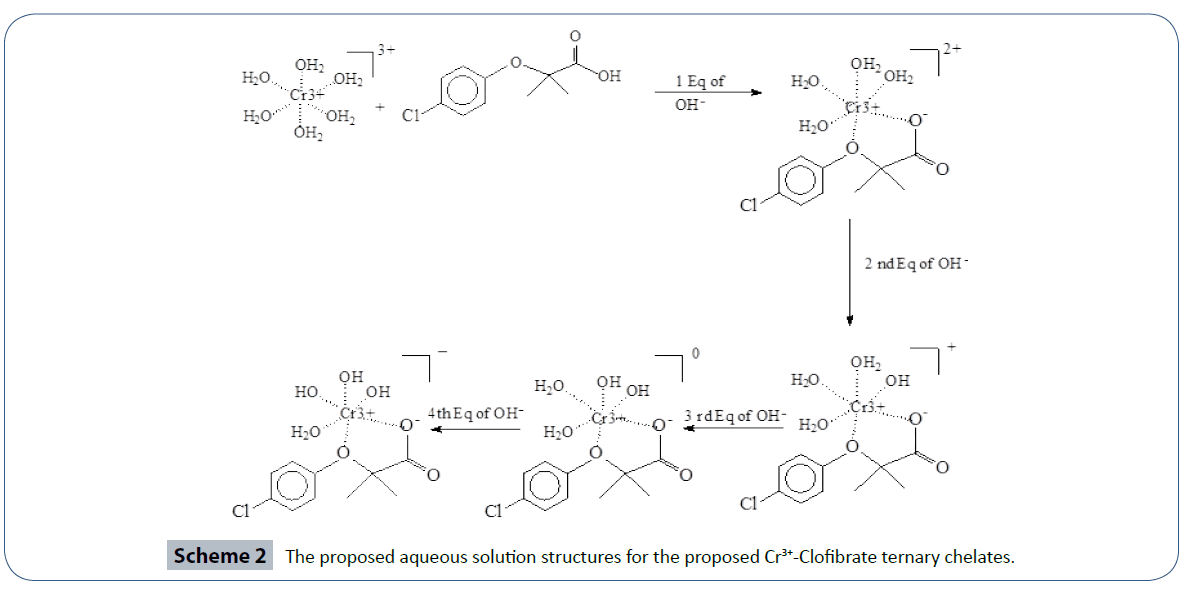
Scheme 2 The proposed aqueous solution structures for the proposed Cr3+-Clofibrate ternary chelates.
Conclusion
The literature evidence overwhelmingly indicated the lack of research articles for CA with essential and toxic metal ions. NIST standard reference database of critically selected protonation constants and stability constants of ligands and their metal complexes does not contain these data for CA [77]. Based on the number of protons released into the solution, we are proposing the formation of the ternary hydroxo-clofibrate metal complex with the formula [Cr3+ (clofibrate-) (OH-)3]1- according to the description in Schemes 2. We believe that the outcome of the data presented in the current report is novel and useful due to the identification of the ternary or mixed Cr3+-Hydroxo- CA complexes in aqueous solutions at room temperature. Also, the reported pKa value in the literature for CA was done in a 50/50 (v/v) acetonitrile/water solvent mixture [81]. The pKa value measured in the current study is in close proximity to the literature value 4.622, reference 81, versus 4.32 of the current study. We have chemically identified a novel ternary Cr3+-CA-OH chelate under ambient conditions. More toxicity and biological studies are needed in these areas to test the effect of these Cr3+ chelate compared to the effect of the free CA alone on PPARα.
Acknowledgement
We would like to acknowledge the financial support from NSF under Grant # HRD-1332459. Special thanks go to LOC faculty for helpful suggestions.
References
- Issemann I, Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645-650.
- Zoete V, Grosdidier A, Michielin O (2007) Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim. Biophys. Acta1771: 915-925.
- VandenHeuvel JP (2007) The PPAR resource page. BiochimBiophysActa 1771: 1108-1112.
- Badr M1 (2006) A forum for a highly important and ever-expanding field of study. PPAR Res 2006: 61385.
- Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta.ProcNatlAcadSci U S A 94: 4312-4317.
- Bishop-Bailey D (2000) Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol 129: 823-834.
- Yokoyama Y, Xin B, Shigeto T, Umemoto M, Kasai-Sakamoto A, et al. (2007) Clofibric acid, a peroxisome proliferator-activated receptor alpha ligand, inhibits growth of human ovarian cancer. Mol Cancer Ther 6: 1379-1386.
- Vohl MC, Badr M, Wieczorek S (2009) Genetic Variation of PPARs.PPAR Res 2009: 189091.
- Giampietro L, Ammazzalorso A, Giancristofaro A, Lannutti, F, Bettoni G, et al. (2009) Synthesis and Biological Evaluation of 2-Heteroarylthioalkanoic Acid Analogues of Clofibric Acid as Peroxisome Proliferator-Activated Receptor Agonists. J Med Chem 52: 6224–6232.
- Moncol J, Kalinakova B, Svorec J,Kleinova M, Koman M, HudecovaD, et al. (2004) Spectral properties and bio-activity of copper(II) clofibriates, part III Crystal structure of Cu(clofibriate)2(2-pyridylmethanol)2,Cu(clofibriate)2(4-pyridylmethanol)2(H2O) dihydrate, and Cu2(clofibriate)4(N,N diethylnicotinamide)2. Inorg. Chim. Acta 357: 3211-3222.
- Ghauch A, AbouAssi H, Tuqan A (2010) Investigating the mechanism of clofibric acid removal in Fe(0)/H2O systems. J Hazard Mater 176: 48-55.
- Brown AD, Davis RD, Fitzgerald RN, Glover BN, Harvey KA, et al. (2009) Development for Sodelglitazar: A PPAR Panagonist. Org Process Res Dev 13: 297-302.
- Rosse G (2013) Diphenylpropane Derivatives as Agonist of PPAR Nuclear Receptors.ACS Med ChemLett 4: 1135-1136.
- Li JL, Xiao B, Park M, Yoo ES, Shin S, et al. (2012) PPAR-γ agonistic metabolites from the ascidian Herdmaniamomus. J Nat Prod 75: 2082-2087.
- Dunny E, Evans P (2010) Stereocontrolled synthesis of the PPAR-gamma agonist 10-nitrolinoleic acid. J Org Chem 75: 5334-5336.
- Karmakar R, Mal D (2012) Total synthesis of chlorocyclinone A, a PPAR-γ antagonist. J Org Chem 77: 10235-10248.
- Deussen H, Jeppesen L, Schärer N, Junager F, Bentzen B Weber (2004) Process Development and Scale-Up of the PPAR Agonist NNC 61-4655. Org. Proc. Res. Dev 8: 363–371.
- Kline BJ, Saenz J,Stankovic N, Mitchell MB (2006) Polymorph and Particle Size Control of PPAR Compounds PF00287586 and AG035029. Org Process Res Dev 10: 203–211.
- Lee HW, Ahn JB, Kang SK, Ahn SK, Ha DC, (2007) Process Development and Scale-Up of PPAR a/? Dual Agonist Lobeglitazone Sulfate (CKD-501). Org. Process Res. Dev 11: 190–199.
- Oh LM, Wang H, Shilcrat SC, Herrmann RE, Patience D B (2007) Development of a Scalable Synthesis of GSK183390A, a PPAR a/? Agonist. Org Process Res Dev 11: 1032–1042.
- Hsu WH, Lee BH, Hsu YW, Pan TM (2013) Inhibition of Th2 cytokine production in T cells by monascin via PPAR-γ activation. J Agric Food Chem 61: 8126-8133.
- Ngan NT, Quang TH, Tai BH, Song SB, Lee D, et al. (2012) Anti-inflammatory and PPAR transactivational effects of components from the stem bark of Ginkgo biloba.J Agric Food Chem 60: 2815-2824.
- Salt DW, Hudson BD, Banting L, Ellis MJ, Ford MG (2005) DASH: a novel analysis method for molecular dynamics simulation data. Analysis of ligands of PPAR-gamma. J Med Chem 48: 3214-3220.
- Nevin DK, Peters MB, Carta G, Fayne D, Lloyd DG (2012) Integrated Virtual Screening for the Identification of Novel and Selective Peroxisome Proliferator-Activated Receptor (PPAR) Scaffolds. J Med Chem55: 4978-4989.
- Potterat O, Puder C, Wagner K, Bolek W, Vettermann R, et al. (2007) Chlorocyclinones A-D, chlorinated angucyclinones from Streptomyces sp. strongly antagonizing rosiglitazone-induced PPAR-gamma activation.J Nat Prod 70: 1934-1938.
- Li JL, Xiao B, Park M, Yoo ES, Shin S, et al. (2012) PPAR-γ agonistic metabolites from the ascidian Herdmaniamomus. J Nat Prod 75: 2082-2087.
- Azimzadeh O, Sievert W, Sarioglu H, Yentrapalli R, Barjaktarovic Z, et al. (2013) PPAR alpha: a novel radiation target in locally exposed Musmusculus heart revealed by quantitative proteomics. J Proteome Res 12: 2700-2714.
- Kuwabara N, Oyama T, Tomioka D,Ohashi M, Yanagisawa J (2012) Peroxisome Proliferator-Activated Receptors (PPARs) Have Multiple Binding Points That Accommodate Ligands in Various Conformations: Phenylpropanoic Acid-Type PPAR Ligands Bind to PPAR in Different Conformations, Depending on the Subtype. J Med Chem 55: 893–902.
- Ye Q, Huang Y, Rusowicz A, Palaniswamy VA, Raglione TV (2010) Understanding and Controlling the Formation of an Impurity during the Development of Muraglitazar, a PPAR Dual Agonist. Org Process Res Dev 14: 238-241.
- Houpis IN, Patterson LE, Alt CA, Rizzo JR, Zhang TY, et al. (2005) Synthesis of PPAR Agonist via Asymmetric Hydrogenation of a Cinnamic Acid Derivative and Stereospecific Displacement of (S)-2-Chloropropionic Acid Org Lett 7: 1947-1950.
- Ji CG, Zhang JZ (2008) Protein polarization is critical to stabilizing AF-2 and helix-2' domains in ligand binding to PPAR-gamma. J Am ChemSoc 130: 17129-17133.
- Tu Z, Moss-Pierce T, Ford P, Jiang TA (2013) Rosemary (Rosmarinusofficinalis L.) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. J Agric Food Chem 61: 2803-2810.
- Markt P, Petersen RK,Flindt EN, Kristiansen K, Kirchmair J,et al. (2008) Discovery of Novel PPAR Ligands by a Virtual Screening Approach Based on Pharmacophore Modeling, 3D Shape, and Electrostatic Similarity Screening. J Med Chem 51: 6303-6317.
- Scatena R, Bottoni P, Vincenzoni F, Messana I, Martorana GE, et al. (2003) Bezafibrate induces a mitochondrial derangement in human cell lines: a PPAR-independent mechanism for a peroxisome proliferator.Chem Res Toxicol 16: 1440-1447.
- Lee YK, Lee WS, Hwang JT, Kwon DY, Surh YJ, et al. (2009) Curcumin exerts anti-differentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and anti-proliferatory effect through AMPKalpha-COX-2 in cancer cells.J Agric Food Chem 57: 305-310.
- Shih PH, Hwang SL, Yeh CT, Yen GC (2012) Synergistic Effect of Cyanidin and PPAR Agonist against Nonalcoholic Steatohepatitis-Mediated Oxidative Stress-Induced Cytotoxicity through MAPK and Nrf2 Transduction Pathways. J Agric Food Chem 60: 2924–2933.
- Lohray BB, Lohray VB, Bajji AC, Kalchar S, Poondra RR, et al. (2001) (-)3-[4-[2-(Phenoxazin-10-yl)ethoxy]phenyl]-2-ethoxypropanoic acid [(-)DRF 2725]: a dual PPAR agonist with potent antihyperglycemic and lipid modulating activity. J Med Chem 44: 2675-2678.
- Rudolph J, Chen L,Majumdar D, Bullock WH, Burns M,et al. (2007)Indanylacetic Acid Derivatives Carrying 4-Thiazolyl-phenoxy Tail Groups, a New Class of Potent PPAR a/?/d Pan Agonists:? Synthesis, Structure-Activity Relationship, and In Vivo Efficacy. J Med Chem 50: 984-1000.
- Cvetovich RJ, Chung JY, Kress MH, Amato JS, Matty L, et al. (2005) An efficient synthesis of a dual PPAR alpha/gamma agonist and the formation of a sterically congested alpha-aryloxyisobutyric acid via a Bargellini reaction.J Org Chem 70: 8560-8563.
- Aikins JA, Haurez M, Rizzo JR, Van Hoeck JP, Brione, WKestemont, et al. (2005) Synthesis of a Peroxime Proliferator Activated Receptor (PPAR) a/? Agonist via Stereocontrolled Williamson Ether Synthesis and Stereospecific SN2 Reaction of S-2-Chloro Propionic Acid with Phenoxides. J Org Chem 70: 4695-4705.
- Kuo MY, Ou HC, Lee WJ, Kuo WW, Hwang LL, et al. (2011) Ellagic Acid Inhibits Oxidized Low-Density Lipoprotein (OxLDL)-Induced Metalloproteinase (MMP) Expression by Modulating the Protein Kinase C-a/Extracellular Signal-Regulated Kinase/Peroxisome Proliferator-Activated Receptor?/Nuclear Factor-?B (PKC-a/ERK/PPAR-?/NF-?B) Signaling Pathway in Endothelial Cells. J Agric. Food Chem 59: 5100–5108.
- Scheytt T, Grams S, Rejman-Rasinski E, Heberer T, Stan HJ.Pharmaceuticals in Groundwater: Clofibric Acid beneath Sewage Farms South of Berlin, Germany. ACS Symposium Series 791: 84–99.
- Sallustio BC, Degraaf YC, Weekley JS, Burcham PC (2006) Bioactivation of carboxylic acid compounds by UDP-Glucuronosyltransferases to DNA-damaging intermediates: role of glycoxidation and oxidative stress in genotoxicity. Chem Res Toxicol 19: 683-691.
- Southwood HT, DeGraaf YC, Mackenzie PI, Miners JO, Burcham PC, et al. (2007) Carboxylic acid drug-induced DNA nicking in HEK293 cells expressing human UDP-glucuronosyltransferases: role of acyl glucuronide metabolites and glycation pathways. Chem Res Toxicol 20: 1520-1527.
- Tixier C, Singer HP,Oellers S, Müller S R (2003) Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol 37: 1061-1068.
- Matamoros V, GarcÃa J, Bayona JM (2005) Behavior of selected pharmaceuticals in subsurface flow constructed wetlands: a pilot-scale study.Environ SciTechnol 39: 5449-5454.
- Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, et al. (2002) Removal of pharmaceuticals during drinking water treatment. Environ SciTechnol 36: 3855-3863.
- Razavi B, Song W, Cooper WJ, Greaves J, Jeong J (2009) Free-radical-induced oxidative and reductive degradation of fibrate pharmaceuticals: kinetic studies and degradation mechanisms. J PhysChemA 113: 1287-1294.
- Kunkel U, Radke M (2011) Reactive tracer test to evaluate the fate of pharmaceuticals in rivers.Environ SciTechnol 45: 6296-6302.
- Wang S, Holzem R M, GunschC K (2008) Effects of Pharmaceutically Active Compounds on a Mixed Microbial Community Originating from a Municipal Wastewater Treatment Plant. Environ SciTechnol42: 1091-1095.
- Azzouz A, Jurado-Sánchez B, Souhail B, Ballesteros E (2011) Simultaneous Determination of 20 Pharmacologically Active Substances in Cow's Milk, Goat's Milk, and Human Breast Milk by Gas Chromatography–Mass Spectrometry. J Agric Food Chem 59: 5125–5132.
- Buser HR, Müller MD (1998) Occurrence of the Pharmaceutical Drug Clofibric Acid and the Herbicide Mecoprop in Various Swiss Lakes and in the North Sea. Environ SciTechnol 32: 188-192.
- Loffler D, Rombke J, Meller M, Ternes TA (2005) Environmental fate of pharmaceuticals in water/sediment systems.Environ SciTechnol 39: 5209-5218.
- Daughton CG (1048) Pharmaceutical Ingredients in Drinking Water: Overview of Occurrence and Significance of Human Exposure. ACS Symposium Series 1048: 9-68.
- Báthori NB, Lemmerer A, Venter GA, Bourne SA, Caira MR (2011) Pharmaceutical Co-crystals with Isonicotinamide—Vitamin B3, Clofibric Acid, and Diclofenac—and Two Isonicotinamide Hydrates. Cryst Growth Des 11: 75–87.
- Sidenius U, Skonberg C, Olsen J, Hansen SH (2004) In vitro reactivity of carboxylic acid-CoA thioesters with glutathione. Chem Res Toxicol 17: 75-81.
- Calderon-Preciado D, Renault Q, Matamoros V, Canameras N, Bayona JM (2012) Uptake of organic emergent contaminants in spath and lettuce: an in vitro experiment.J Agric Food Chem 60: 2000-2007.
- Kimura K, Hara H, Watanabe Y (2007) Elimination of Selected Acidic Pharmaceuticals from Municipal Wastewater by an Activated Sludge System and Membrane Bioreactors. Environ SciTechnol 41: 3708–3714.
- Li W, Lu S, Qiu Z, Lin K (2011)Photocatalysis of Clofibric Acid under Solar Light in Summer and Winter Seasons. IndEngChem Res 50: 5384-5393.
- Montanari R, Saccoccia F, Scotti E, Crestani M, Godio C, et al. (2008) Crystal Structure of the Peroxisome Proliferator-Activated Receptor ? (PPAR?) Ligand Binding Domain Complexed with a Novel Partial Agonist: A New Region of the Hydrophobic Pocket Could Be Exploited for Drug Design. J Med Chem51: 7768–7776.
- Radke M, Ulrich H, Wurm C, Kunkel U (2010) Dynamics and attenuation of acidic pharmaceuticals along a river stretch. Environ SciTechnol 44: 2968-2974.
- Hans-Rudolf Buser, Thomas Poiger, Markus D (1999) Occurrence and Environmental Behavior of the Chiral Pharmaceutical Drug Ibuprofen in Surface Waters and in Wastewater. Müller Environ SciTechnol 33: 2529–2535.
- Corcoran J, Lange A, Winter MJ, Tyler CR (2012) Effects of pharmaceuticals on the expression of genes involved in detoxification in a carp primary hepatocyte model. Environ SciTechnol 46: 6306-6314.
- Cécile Michel, Ruth A Roberts, Chantal Desdouets, Kevin R Isaacs, Eric Boitier (2005) Characterization of an Acute Molecular Marker of Nongenotoxic Rodent Hepatocarcinogenesis by Gene Expression Profiling in a Long Term Clofibric Acid Study. Chem ResToxicol18: 611–618.
- Grillo MP, Benet LZ (2001) Interaction of gamma-glutamyltranspeptidase with clofibryl-S-acyl-glutathione in vitro and in vivo in rat.Chem Res Toxicol 14: 1033-1040.
- Bailey MJ, Dickinson RG (1996) Chemical and immunochemical comparison of protein adduct formation of four carboxylate drugs in rat liver and plasma. Chem Res Toxicol 9: 659-666.
- Cowan JA (1997) In Inorganic Biochemistry/An introduction, Wiley-VCH Inc. Hoboken, NJ.
- Tietz NW (1994) textbook of clinical chemistry, (2nd edn), Carl A. Burtis and Edward R. Ashwood Ed. Saunders, Philadelphia, PA.
- Vincent JB (1999) Mechanisms of chromium action: low-molecular-weight chromium-binding substance. J Am CollNutr 18: 6-12.
- Yamamoto A, Wada O, Ono T (1987) Isolation of a biologically active low-molecular-mass chromium compound from rabbit liver. Eur J Biochem 165: 627-631.
- Hamada YZ, Carlson B,Dangberg (2005)Interaction of malate and lactate with chromium(III) and iron(III) in aqueous solutions, SynReacInorg Met-Org Nano-Met Chem 35: 515-522.
- Hamada YZ, Holyfield H, Rosli K, Burkey TJ, (2009) Equilibrium models of Cr3+ and Cu2+ with glutamate, CoordChem62: 721-733.
- Hamada YZ, Carlaon BL, Shank JT (2003) Potentiometric and UV-Vis spectroscopy studies of citrate with the hexaquo Fe3+ and Cr3+ metal ions, SynReacInorg Metal-Org Chem 33: 1425-1440.
- Hamada YZ, Bayakly N, Peipho A, Carlson B (2006) Accurate potentiometric studies of Chromium-citrate and ferric citrate complexes in aqueous solutions at physiological and alkaline pH-values, SynReacInorg Met-Org Nano-Met Chem 36: 469-476.
- Alderighi L, Gans P, Ienco A, Perters D, Sabatini A, et al. (1999)Hyperquad simulation and speciation (Hyss): a utility program for the investigation of equilibria involving soluble and partially soluble species, CoordChem Rev 184: 311-318.
- Sweeton FH, Mesmer RE, BaesJr CF (1974) Acidity measurements at elevated temperature. VII. Dissociation of water. J Sol Chem 3: 191-214.
- Martell AE, Smith RM, Motekaitis RJ (2001) Critical Stability Constants Database, Version 6.0, (2001) NIST, Texas A & M University, College Station, TX, USA.
- Kettle SFA (1996) Physical Inorganic Chemistry, A Coordination Chemistry Approach, Spektrum. University Science Book, Sausalito, CA.
- Wiley and Sons (1967) The hydrolysis of cations. Baes CF, Mesmer RE. New York.
- Hamada YZ, Harris WR, Rath N (2013) Crystal Structure of Pyridoxal Amino Methyl Phosphonic Acid (PYRAMPA) and Its Stability Constants with Al3+. International J. Green and Nano Tech 1-8.
- Canbay HS, Demiralay EC, Alsancak G, Ozkan SA (2011) Chromatographic Determination of pKa Values of Some Water-Insoluble Arylpropionic Acids and Arylacetic Acids in Acetonitrile+Water Media. JChem Eng Data 56: 2071–2076.