- (2014) Volume 15, Issue 2
Sirio Fiorino1, Letizia Bacchi-Reggiani2, Laura Pontoriero4, Claudio Gallo5, Elisabetta Chili6, Michele Masetti7, Nicola Zanini7, Ana Grondona8, Tania Silvestri8, Gaia Deleonardi8, Adele Fornelli9, Arrigo Bondi9, Dario de Biase10, Paola Baccarini10, Giovanni Tallini10, Antonio Tropeano11, Valeria Quartuccio12, Andrea Cuppini1, Gastone Castellani3, Elio Jovine7
1Unit of Internal Medicine, Hospital of Budrio. Budrio, BO, Italy.
2Institute of Cardiology, “S. Orsola-Malpighi Hospital”, and
3Institute of Fisics; “Alma Mater Studiorum” - University of Bologna. Bologna, Italy.
4Unit of Mother-Child Care, Lametino District, ASP. Catanzaro, Italy.
5Physician Specialist of Infectious Diseases.
6Unit of Anestesiology, Modena Hospital, University of Modena. Modena, Italy.
7Unit of General Surgery A, 8Central Laboratory, and 9Department of Pathology; “Maggiore” Hospital. Bologna, Italy.
10Department of Pathology, Bellaria Hospital. Bologna, Italy.
11Physician Specialist of Dentistry.
12Calabria University. Cosenza, Italy.
Received November 16th, 2013 – Accepted December 24th, 2013
Context Hepatitis B (HBV) and hepatitis C virus (HCV) possess well-known oncogenic properties and may promote carcinogenesis in liver. However antigens and replicative sequences of HBV/HCV have been also detected in different extrahepatic tissues, including the pancreas. Although epidemiological studies and meta-analyses have recently suggested that HBV/HCV may be also risk factors for pancreatic cancer and several researches have investigated the possible mechanisms and intra-/extra-cellular paths involved in pancreatic and hepatic carcinogenesis, to date, these complex processes remain largely unexplained. Objectives In our paper, we aimed to propose a comprehensive and qualitative hypothetical model, describing how HBV/HCV may exert their oncogenic role. Methods We performed a systematic research of scientific literature, by searching MEDLINE, the Cochrane Library and EMBASE databases. The used keywords were: “chronic HBV/HCV”, “pancreatic cancer”, “liver carcinoma”, “carcinogenesis mechanisms”, “tensional integrity”, “cytoskeleton”, and “extracellular matrix”. Results Taking advantage from available studies, we suggest an unifying hypothesis based on results and data, obtained from different areas of research. In particular we considered the well-defined model of tensional integrity and correlated it to changes induced by HBV/HCV in viscoelastic properties/stiffness of cellular/extracellular microenvironments. These events perturb the tightly-regulated feedback loop, which usually couples the intracellulargenerated forces to substrate rigidity of extracellular compartments. Therefore, such a change strongly affects intracellular functions and cellular fate, by promoting a substantial deregulation of critical intracellular biochemical activities and genome expression. Conclusions Our hypothesis might provide for the first time a reliable system, which correlates tensional integrity model with intra-/extra-cellular modifications, occurring in liver and pancreas during HBV/HCV-induced carcinogenesis. This approach might improve our understanding of pathogenetic mechanisms involved in the development of pancreatic and hepatic carcinogenesis , enhancing the possibility of their treatment. Furthermore, should the usefulness of this model be definitively confirmed, it might be also helpful to extend its field of application to other viruses-related cancers.
Carcinoma, Hepatocellular; Hepacivirus; Hepatitis B virus; Mechanotransduction, Cellular; Pancreatic Carcinoma
ECM: extracellular matrix; ERK: extracellular signal-regulated kinase; FAK: focal adhesion tyrosine-kinase; HBV: hepatitis B virus; HBx: hepatitis B x protein; HCV: hepatitis C virus; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein-kinase; MEK: mitogen-activated protein kinase kinase kinases; NS protein: non-structural protein; Src: Src family kinases
Hepatitis B virus (HBV) and hepatitis C virus (HCV) persistent infections represent a serious public health problem worldwide. Although considerable variations exist among different countries and geographical areas, it is estimated that about 5% of the world population is chronically infected with HBV, corresponding to approximately 350-400 million people, whereas the global prevalence of HCV is 2.8%, with nearly 180 million people affected [1, 2]. Both viruses may cause a necroinflammatory hepatic disease with different patterns of severity and course and it is well-known that persistent liver injury represents a high risk condition for developing cirrhosis and hepatocellular carcinoma [3, 4]. Furthermore, some cohort and case/control studies [5, 6, 7, 8, 9, 10], metaanalyses [11, 12, 13] and a recent report [14], demonstrating that HBV is able to infect and replicate in pancreatic cancerous tissue, strongly suggest that HBV and HCV may represent also risk factors for the development of adenocarcinoma in this organ. Although a large series of studies have been performed in the past to improve our knowledge of events promoting hepatic and pancreatic carcinogenesis, to date, pathogenetic mechanisms, involved in tumor initiation, progression and growth in both organs, are not completely understood.
In the last years, available researches reported that the existence of a persistent inflammation is a condition predisposing to cancer development in tissues, where this process occurs [15, 16, 17]. In particular, it is well-known that patients suffering not only from chronic hepatitis and pancreatitis, but also from Barrett’s esophagus and inflammatory bowel diseases, present an increased risk of cancer in comparison with controls. According to epidemiological and experimental data, it has been shown that several viruses, including HBV, HCV, human immunodeficiency virus, Epstein-Barr virus, human papilloma virus and human herpes virus 8, possess oncogenic properties. It has been suggested that the role of these viruses in human carcinogenesis depends on their ability to promote a process of chronic inflammation in infected tissues. Approximately 15-20% of all malignancies are associated with persistent infection. However, inflammation might not be the only mechanism involved in cancer development. Other mechanisms, alone or in cooperation with inflammation, could promote cancer development and contribute substantially to tumor initiation and progression.
The purpose of this work is to discuss the hypothesis that oncogenic role of HBV and HCV is related to their ability to induce a direct deregulation of normal balance between intracellular and extracellular tensional forces generated by cytoskeleton and extracellular matrix adhesions respectively. It has been reported that perturbation of this interplay causes a substantial change of critical intracellular biochemical activities as well as of genome expression, influencing cellular fate. Irrespective of the well-known role of inflammatory processes in cancer development, both viruses might also promote carcinogenesis in infected cells via alteration of the tightly regulated mechanisms, which regulate the interactions between intracellular cytoskeleton and extracellular matrix. Therefore, we consider the “tensegrity model hypothesis”, developed several years ago by Donald E Ingber to explain mechano-biology and diseases of mechano-transduction, and we provide an hypothetical mechanisms involved in HBV- and HCV-related tumor initiation and promotion.
With the aim to evaluate this hypothesis, we performed a comprehensive systematic research of scientific literature and we considered the following electronic databases: MEDLINE (1950 to July 30th, 2013), the Cochrane Library (until the first quarter of 2013) and EMBASE (1980 to July 30th, 2013) for all potential relevant articles.
Two authors (C.G., E.C.) independently performed the literature search, identified and screened relevant articles, based on title and abstract. The text of the potentially relevant articles was retrieved for further evaluation.
The search text words were identified by means of controlled vocabulary. The used MeSH terms were: “HBV/HCV”, “pancreatic cancer”, “liver cancer”, “carcinogenesis mechanisms”, “tensional integrity”, “cytoskeleton”, and “extracellular matrix”. These keywords were associated according to the following scheme: HBV and pancreatic cancer (45 citations); HCV and pancreatic cancer (44 citations); HBV and carcinogenesis mechanisms (81 citations); HCV and carcinogenesis mechanisms (66 citations), HBV and cytoskeleton (32 citations), HCV and cytoskeleton (36 citations), tensegrity (168 citations), HBV and tensegrity (0 citations); HCV and tensegrity (0 citations); HBV and extracellular matrix (40 citations); HCV and extracellular matrix (85 citations); HBV and liver cancer (4,467 citations); HCV and liver cancer (3,785 citations); tensegrity and liver cancer (0 citations), tensegrity and pancreatic cancer (0 citations); extracellular matrix and tensegrity (13 citations); tensegrity and carcinogenesis mechanisms (0 citations); extracellular matrix and carcinogenesis mechanisms (127 citations); extracellular mechanisms and cytoskeleton (1,325 citations). The PubMed “related articles” feature and the reference lists of retrieved articles were also searched to find additional pertinent studies. The search was restricted to peer-reviewed, full-text publications, written in English. Studies that were not published as full reports, such as conference abstracts or case reports, were excluded. Two hundred and sixtyeight potentially relevant articles were identified and considered in this review. A selection of these papers was also reported in the reference list. Because of characteristics of identified researches, no quantitative assessment of these studies was possible.
Taking advantage from data reported in identified articles, the conclusions of researches were summarized and organized into the following six sections by two further authors (T.S., G.D.). The results of selected studies were used to propose a systematic and comprehensive model, describing how HBV/HCV might exert their oncogenic role in hepatic and pancreatic carcinogenesis.
In particular, in our review we synthesized and reported:
1) the studies describing the normal organization of intracellular cytoskeleton as well as of extracellular matrix (ECM);
2) the researches examining the “tensional integrity” or “tensegrity” model and explaining how some crucial cellular functions, such as differentiation, apoptosis or growth are modulated by cellular architecture and shape;
3) the evidences evaluating how modifications of cytoskeletal framework, as well as of extracellular structure, influence mechanical properties of cells and extracellular matrix, such as cellular contractility and matrix stiffness;
4) the reports showing perturbation in production, release and deposition of matrix proteins in liver and pancreas, during the process of pancreatic and hepatic carcinogenesis;
5) the studies reporting the interactions between HBV or HCV related proteins, such as hepatitis B x protein (HBx) and non-structural (NS) 3, NS5A, NS5B proteins and several elements of cellular cytoskeleton of hepatocytes and stellate cells, as well as between HBx/NS5A and different components of extracellular matrix;
6) the possible mechanisms involved in HBV- and HCV-mediated perturbation of tissue “tensegrity”, inducing changes in intracellular biochemistry and gene expression, causes changes in intracellular biochemistry and gene expression.
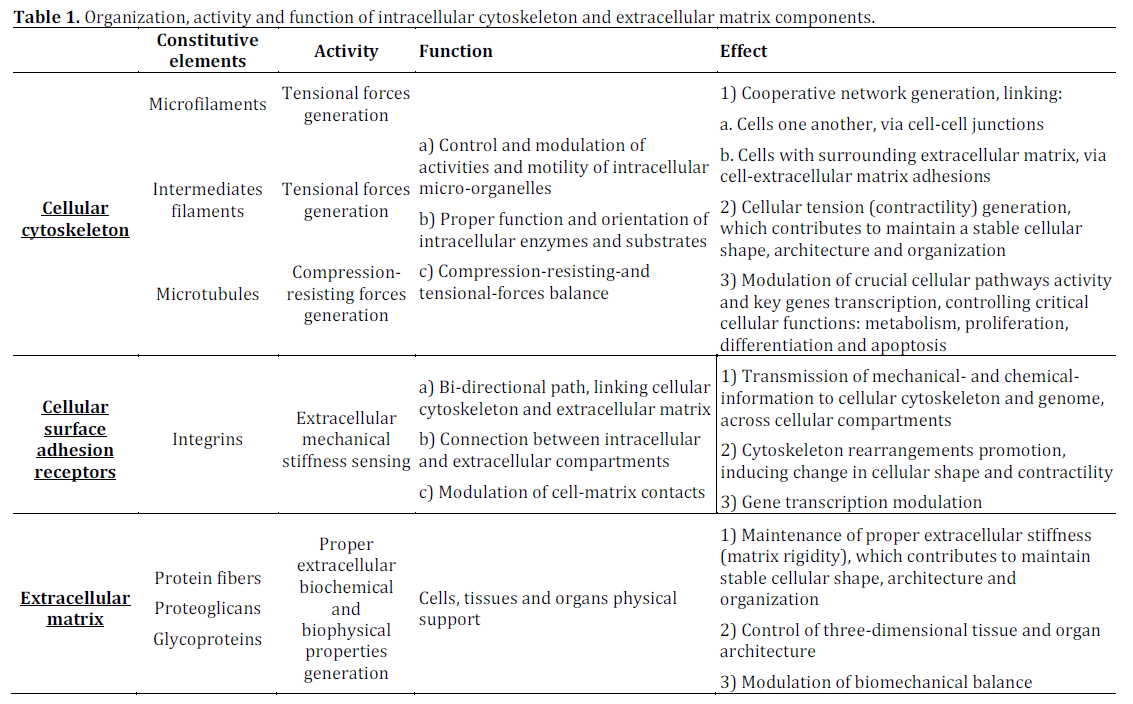
Normal Organization of Intracellular Cytoskeleton and Extracellular Matrix
All nucleated cells possess a special structure, known as the “cytoskeleton”, a molecular framework that generates tensile forces and distribute them to other cells, as well as to extracellular components [18]. Cytoskeleton is the most important mechanical support within the cells. It consists of three major interconnected elements, represented by microfilaments, intermediate filaments and microtubules, anchored to specialized adhesion structures both at cell-cell junctions [19, 20] and at cell-ECM attachment points [21]. This intricate architecture provides a link between extraand intra-cellular microenvironment and several signaling pathways are implicated in the coordinate activity of this complex network. Cytoskeleton is a scaffold which controls and modulates motility and activities of cellular micro-organelles and proper function and orientation of enzymes and substrates, with a key role in critical biochemical reactions within cells [22]. Integrins are the most important cellular surface adhesion receptors, which mediate and modulate cell-matrix contacts [23]. Their structure consists of different components, including extracellular-, intra-membrane- and cytoplasmic-domains [24]. Integrins are lacking of enzymatic function and a large series of intracellular proteins is associated with these receptors and cooperate to optimize their activity. In particular, binding of cells to ECM causes clustering of integrins on cellular surface, an early phase of the multistep process, occurring during the formation of these specialized junction structures, termed “focal adhesions” [25, 26]. This event produces the recruitment of different types of proteins to the inner surface of cellular membrane. The adaptor/scaffold proteins involved in the proper formation of nascent cellular focal adhesions include talin, paxillin, tensin, vinculin, p130Cas and α-actinin [27, 28, 29], they contribute to form a suitable link between cytoskeleton and ECM [30]. Then, α-actinin triggers adhesion structure maturation, via linking integrins to actin cytoskeletal filaments, promoting cytoskeleton rearrangements and generation, as well as transmission of tensional forces [31]. Furthermore, several enzymes, including focal adhesion tyrosinekinase (FAK), c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase kinase kinases (MEK), extracellular signal-regulated kinase (ERK) and non-receptor tyrosine kinases family, known as Src family kinases (Src), are also concentrated to focal adhesions, where they transfer ECMoriginated stimuli to cellular signaling cascades. Several components of the Rho-family of small GTPases represent additional important enzymes, modulating this complex process, via control of actin polymerization and cytoskeletal tension generation [32]. These proteins also localize in cellmatrix adhesions and in association with other kinases, in particular with Src family kinases, contribute to control several critical intracellular signaling paths, that regulate crucial cellular functions, such as motility, growth, proliferation, differentiation and survival [33]. Depending on different organization of intracellular environment, cytoskeleton may have a dual activity, originating either compressive or tensional forces. In particular, intracellular microfilaments, as well as intermediate filaments, develop tension and balance compression-resisting forces, which are generated by microtubules and extracellular matrix adhesions [34].
Therefore, cytoskeleton may be considered as a dynamic frame that in cells plays a key role in establishing a constant organization and in maintaining both a stable shape and a regular architecture. In addition, cytoskeleton constitutes a cooperative, although intricate, network, which contributes to link cells one another, via cell-cell junctions, and with surrounding ECM, via cell-ECM adhesions (Figure 1; Table 1). This connection occurs either by means of membrane basement interposition or via a direct association with exogenous matrix. Thus, cells do not represent separate or distinct elements, but they form a wellinterconnected and integrated frame [33]. In particular, extracellular matrix consists of several components, including protein fibers (collagen and elastin) which are kept together by carbohydrate strings, such as proteoglycans and glycoproteins. Proteoglycans are widely distributed in connective tissues and exert important activities. It has been shown that proteoglycans may control several steps of collagen production and deposition, modulating synthesis of fibrils and their diameter, as well as influencing physical and functional properties of tissues, via mechanical coupling among collagen fibers [35, 36]. Therefore, proteoglycans function as structural elements, regulating tissue organization, modulate water balance of ECM, interact with cytokines and growth factors; furthermore, they contribute to control tissue biomechanical balance and play a crucial role in tissue repair, development and maintenance of homeostasis [37, 38].
Figure 1. Schematic representation of signaling pathways activated by direct or indirect applications of forces to cellular membrane and/or extracellular matrix, via integrin-anchored focal adhesion structures. Integrins mediate cell-matrix contacts, form a suitable link between cytoskeleton and ECM and provide a bi-directional route to connect the intracellular and extracellular compartments. Tensional forces, focused within focal adhesion structures, induce clustering of integrin receptors. Integrin engagement cause recruitment of signaling proteins, such as talin, paxillin, tensin, paxillin and α-actinin, providing a connection with actin cytoskeletal filaments, microfilaments, intermediate filaments and microtubules. This event promotes cytoskeleton rearrangement. Furthermore, several enzymes, including focal adhesion tyrosine-kinase (FAK), c-Jun N-terminal kinase (JNK), mitogen-activated protein-kinase (MAPK), mitogen-activated protein kinase kinase kinases (MEK), extracellular signal-regulated kinase (ERK) and non-receptor tyrosine kynases family, known as Src family kinases (Src), are also concentrated to focal adhesions, where they transfer ECMoriginated stimuli to cellular signaling cascades. Several components of the Rho-family of small GTPases represent additional important enzymes, modulating this complex process. In particular Src may directly activate Ras, Raf, MEK, MERK, MAPK, or indirectly, via Crk-associated substrate (CAS) and Rac, nuclear factor kB (NF-kB), JNK and JUN. Furthermore, FAK may activate ERK/MAPK, via recruitment of the growth factorreceptor- bound-2 (GRB-2) and son-of-sevenless (SOS) complex. These events induce chemical and electrical modifications inside the cells, they produce changes in the function of their metabolic machinery and they contribute to modulate genome transcription and protein translation. (Adapted from Ingber DE, 2003 [42])
Normal tissue function depends on stability of cellular shape and ECM architecture, as well as on regular and constant cell-cell connections and cellstroma adhesions [21]. It is well-known that some important cellular activities, including cellular differentiation, apoptosis or growth, are modulated by cellular architecture and shape. In most tissues both cells and ECM concur together to generate an elastic microenvironment. However, the study of cellular responses to different types of biophysical and biochemical stimuli has been generally performed in vitro on cells routinely cultured on a two dimensional substratum. When this approach is used, cells are induced to grow as a monolayer [39]. Nevertheless, this model of study is not able to reproduce the real situation, detectable in viable tissues. Therefore, novel systems of cellular culture have been developed. They are based on the preparation of cells embedded within three dimensional matrices [40, 41]. The use of this model recapitulates the physiological cellular behavior to different degrees and it has allowed to improve our understanding of the mutual relationships, occurring between cells and ECM in vivo, in a more detailed way. Cellular adhesion structures may include several types of integrins, they may recruit distinct subsets of cytoplasmic proteins, which control different cell signaling paths [21, 33]. It has been suggested that the factors inducing the alteration of tissue structural context causes changes of mechanical properties of cells and of surrounding extracellular matrix.
“Tensional Integrity” or “Tensegrity” Model
Some years ago Donald E Ingber proposed the application of the “tensional integrity” or “tensegrity” model to biological structures, with the purpose to explain the complex balance between tensional and compressive forces, existing in intracellular and extracellular compartments [42]. This concept has been gathered by engineering and architecture [43, 44]. According to this theory “tensegrity” architecture is characterized by a tensed network of elements, that self-stabilize their form via continuous tensional generation as well as via association with other support components and that resist compressive forces. Taking advantage from these physical principles, Donald E Ingber applied this model to biological systems. In particular, mechanical burden, which occurs and acts in cells and in stroma, plays a critical role as biological regulator of development and function of tissues and organs [45]. On the basis of “tensegrity” model, cells may be regarded as tensed structures that are able to resist shape distortion and are able to self-stabilize. Cells are able to sense, via integrins, mechanical signals, which are applied to tissues, as well as are able to counteract these stimuli, originating different types of response, such as a change in cytoskeleton organization [45]. This event is generally characterized by an enhancement of cytoskeletal tension, leading to cellular shape modification. Integrins represent a bi-directional path, transmitting mechano-chemical information across cellular membrane and connecting intracellular and extracellular compartments [45]. Cellular deformation, induced by mechanical stimuli, causes not only changes in cytoskeleton framework but also in ECM structure and function [46]. Several factors and mechanisms are involved in these complex processes. The molecular composition of ECM affects its biochemical and mechanical properties and modulates cellular activities. It is well known that qualitative or quantitative changes of matrix components, including modifications of proteoglycans, collagens or glycoproteins considerably impact their biochemical and biophysical characteristics and strongly affect not only extracellular, but also intracellular microenvironments structures and functions [21, 33]. These events promote an increase in stiffness and rigidity of tissues involved. Therefore, according to “tensegrity model” cytoskeleton behaves as an efficient discrete mechanical network.
Modifications of Cytoskeletal Framework and of Extracellular Structure: Their Influence on Mechanical Properties of Cells and Extracellular Matrix
The enhancement of the exogenous stiffness (matrix rigidity) induces key effects on cellular signaling pathways, on matrix adhesion function and on endogenous tension (contractility) [21, 47]. Perturbation of these processes influences cellular fate, by causing a switch of cells among several states critical for tumor initiation and progression, represented by proliferation, apoptosis, differentiation and motility. Therefore, mechanical forces seem to act as regulators of cell and tissue development [48]. Several available researches report that substantial modifications of intracellular signaling paths develop as a consequence of tissue structure change, causing a perturbation of cellular and ECM mechanical properties [49].
The process inducing the conversion of physical forces into biochemical signals is defined mechanotransduction and collective interactions, originating among structures of cytoskeletal framework, influence the form of epithelial cells, mediate their adhesion to membrane basement and contribute to influence cellular fate [22, 49]. The use of “tensegrity model” provides in general a useful concept to explain effectively the transmission of tensional forces from macro- to micro-systems and its application to biological structures represents an effective approach to couple mechanical stresses, originating in extra- and intra-cellular microenvironments, with cytoskeletal rearrangements and reorganization [22, 40]. These events are characterized by chemical and electrical modifications inside the cells and they produce changes in the activity of cellular signaling paths and in the function of metabolic machinery; in addition, they contribute to modulate genome transcription and protein translation. Deregulation of these cellular processes may promote neoplastic transformation and tumor initiation, growth and progression [50].
Deregulation of Production, Release and Deposition of Matrix Proteins in Liver and Pancreas, During the Process of Pancreatic and Hepatic Carcinogenesis
The ECM plays a pivotal role for the maintenance of the normal structure and function of connective tissue, supplying a physical support to cells, tissue and organs and sustaining their three-dimensional architecture [21]. Epidemiological studies suggest that chronic pancreatitis, as well as persistent hepatitis with progressive development of cirrhosis, are strong risk factors for liver and pancreatic carcinoma [1, 51]. The pathological features of these pre-neoplastic conditions are represented by the progressive deposition of an altered ECM, characterized by the production of different amounts and types of interstitial collagens and other elements, such as proteoglycans, glycolproteins and growth factors [21, 33]. Therefore, ECM undergoes a process of substantial remodeling, an event that has a crucial role in the early phases of carcinogenesis in these organs [52]. The modified composition of this supporting framework is associated with critical changes of its physical properties. In particular, the final effect of this complex event is represented by an increased ECM rigidity and stiffness, a condition predisposing to carcinogenesis [53]. Hepatic and pancreatic stellate cells play a crucial role in fibrogenic progression of cirrhosis and chronic pancreatitis as well as in liver and pancreatic cancer induction and progression, via an excessive ECM synthesis and deposition [54, 55]. In normal liver and pancreas, hepatic and pancreatic stellate cells exist in quiescent state as retinoid-containing fat-storing cells. Hepatic stellate cells are localized in the space of Disse in close contact with hepatocytes and endothelial cells of sinusoids in hepatic tissue, whereas pancreatic stellate cells are in proximity to pancreatic periacinar and interlobular regions. During persistent liver and pancreas injury both hepatic and pancreatic stellate cells, undergo a process of activation, acquiring a myofibroblast-like form. The characteristics of their activated phenotype include: loss of vitamin-A droplets; elevated mitotic index and expression of cytoskeletal filament α-smooth muscle actin; production and deposition of matricellular proteins and cytokines; and enhanced contractility and motility [56, 57]. Therefore, a dynamic and self-maintaining cross-talk develops and involves epithelial cells, which are progressively becoming malignant, ECM and a wide spectrum of different soluble interleukins, growth factors and mediators [58]. These events lead to development of a pathological tissue, characterized by the emergence of a hypoxic microenvironment, which promotes the development of fibrosis. Several matricellular proteins are over-expressed or deregulated in different types of human tumors, such as periostin as well as different types of collagens. Periostin expression is enhanced in several cancers [59], including liver and pancreatic carcinomas and its over-production has been associated with increased aggressiveness and poor prognosis of these malignancies. In particular, in normal liver tissues a weak cytoplasmic periostin positivity was detected in hepatocytes and stromal cells. On the other hand, periostin was generally over-expressed in liver foci of hepatocellular carcinoma both in malignant hepatocytes as well as in adjacent stromal cells in comparison to surrounding non-neoplastic [59, 60] or normal liver tissues, with different frequencies [61, 62]. Increased deposition of periostin in hepatic cancerous lesions has been significantly associated with younger age at diagnosis, elevated tumor grade and HBV infection [59] or with cancer nodules, microvascular invasion, Edmondson grade and TNM stage [61]. During pancreatic carcinogenesis also, normal parenchyma is progressively replaced by a desmoplastic tissue rich in collagen, fibronectin, periostin and other proteins. These matrix components support cancer development even in conditions characterized by nutrient deprivation, hypoxia and chemotherapy [63]. In particular, available evidences suggest that tumor stroma predominantly consists of type I collagen and, to a lesser extent, of type III collagen; both these proteins are secreted by pancreatic stellate cells [64]. Furthermore, the integrity of collagen IV-rich basement membrane induces inhibitory effects on ability of epithelial and pancreatic stellate cells to proliferate. The eventual occurrence of gaps in this structure represents a crucial event in tumor progression, because epithelial cells, which are progressively acquiring a transformed phenotype, are placed into direct contact with extracellular matrix proteins, including collagen type I and proteoglycans [65]. It is now known that collagen type I is able to give a survival advantage to pancreatic cancer cells, via reduced expression of Ecadherin, as well as disruption of E-cadherinmediated cell-cell contacts [66]. Therefore, transformed cells acquire an enhanced proliferation and invasiveness ability. In addition, according to available evidences, over-production and deposition of periostin have been reported also in pancreas [60, 63]. It has been shown that this protein is able to promote survival of pancreatic cancer cells, via several mechanisms. In particular, periostin may directly interact with α6β4 integrin complex [60, 63]. The binding of periostin to this cellular surface receptor induces activation of FAK, a critical event that influences cell morphology, motility and adhesion properties [63]. It has been also reported that periostin interacts with several ECM molecules, including fibronectin, tenascin-C and collagen type V [67], as well as it has been shown that periostin is able to directly associate with collagen type I, modulating the process of collagen fibrillogenesis, such as synthesis, assembly and maturation of collagen fibrils. During the process of carcinogenesis, aberrant deposition of periostin and ECM proteins alters viscoelastic and mechanical properties of connective tissues, influencing their rigidity and stiffness [68].
In conclusion, cellular cytoskeleton, extracellular matrices, integrins and Rho kinases constitute an integrated circuit, that is regulated by mechanical forces and that links extracellular microenvironment stiffness to cytoskeletal tension. Therefore, this intricate cross-talk strongly contributes to influence cellular fate and tissue phenotype [33].
Interactions Between HBV or HCV Related Proteins and Different Components of Cellular Cytoskeleton of Hepatocytes and Stellate Cells as Well as Extracellular Matrix
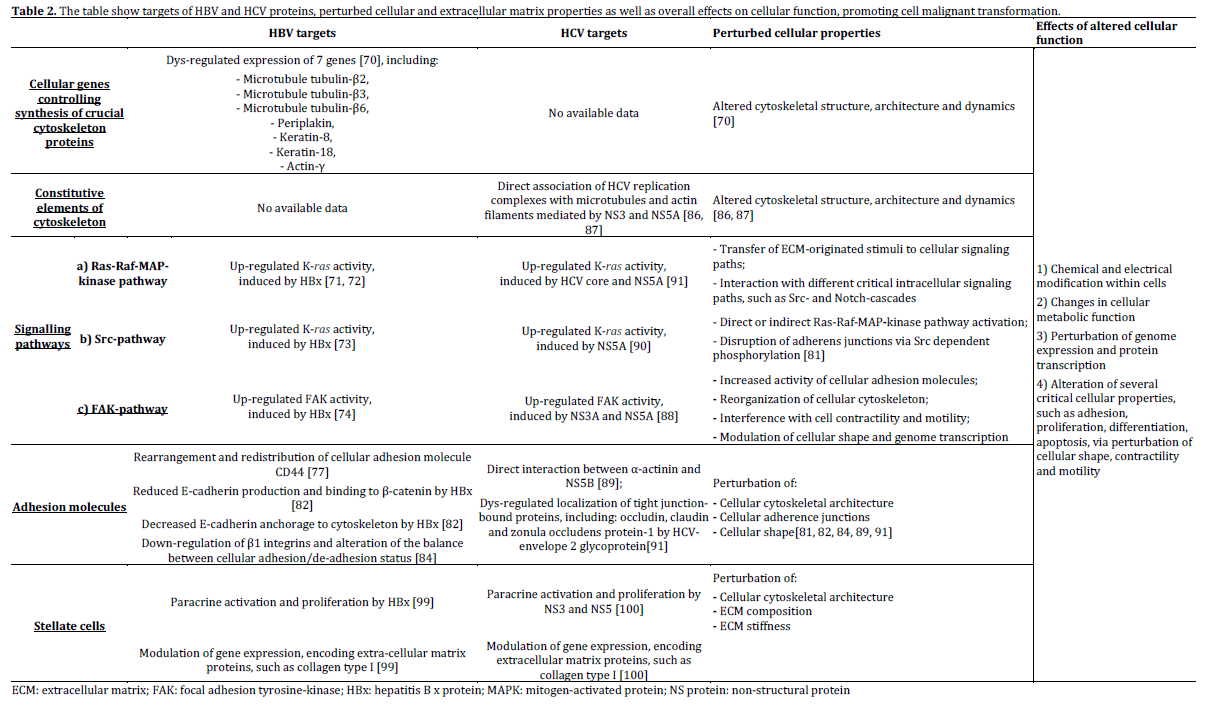
One of the most important events, which occur during chronic viral infections, is represented by the reconfiguration and rearrangements of cellular cytoskeleton. Both processes considerably impact every phase of the viral life cycle, from entry to replication, assembly and egress [69]. In the last years several studies have been performed to examine if direct or indirect interactions may develop among proteins of HBV or HCV and different elements that form the cytoskeleton and extracellular matrix, causing an alteration of its function and, as a consequence, a perturbation of protein signaling networks and activities inside the cell. In spite of considerable efforts to investigate these complex processes, the role of cytoskeletal components in the intracellular life cycle of both viruses is not yet well-known. Remarkable differences exist between HBV and HCV, including genomic structure and transcription/translation paths [2, 3]. However, both viruses share intracellular targets and they might induce an alteration of cytoskeleton structure and function [2, 3]. Different mechanisms may be involved in this process: perturbation of expression profiles of genes encoding different types of cytoskeletal filaments [70], direct selective binding of some viral proteins to target elements of this intracellular scaffold, as well as modulation of the function of cellular proteins, which regulate the activity of several family of tyrosine-kinases. These enzymatic cascades contribute to control distinct components of the cytoskeleton architecture, therefore promoting its dynamic rearrangements and influencing its final organization. HBx for HBV and non-structural 3A (NS3A), 4A (NS4A) and 5A (NS5A) proteins for HCV are the viral elements implicated in the interaction with filaments of intracellular scaffold or in the alteration of cellular cytoskeletal genes expression. Very interesting insights have been obtained in a study performed in p21-HBx transgenic mouse. This research provided the first evidence that HBx may up-regulate the activity of 7 genes, which control the synthesis of specific proteins involved in the organization of different cytoskeletal components. In particular, it has reported that HBx may modulate the function of microtubule and intermediate filament genes. They are respectively represented by microtubule tubulin-β2, tubulin-β3 and tubulin-β6 as well as by periplakin, keratin 8, keratin 18 and actin-γ [70]. Furthermore, several potential direct or indirect targets for proteins of both viruses have been detected on components of cytoskeleton (Figures 2 and 3; Table 2). It has been demonstrated that HBx is able to modulate the activity of numerous transcription factors, which regulate cellular signalling transduction paths, such as Ras-Raf-MAP kinase [71, 72] and Src family kinase [73] cascades. In particular, it has been reported that this viral protein activates Ras via enhanced uptake of GTP onto Ras. According to some studies, HBx may promote or facilitate the association of some adaptor proteins, such as Shc, Grb2 and Sos, but without generating a complex with them [72]. Furthermore, it has been observed that HBx is able to stimulate the activity of focal adhesion tyrosinekinase FAK in HepG2 cells [74]. This enzyme is detectable in cellular focal contacts and it represents one of the members of cytoskeletal framework. The activation of FAK results in phosphorylation-dependent increased function of multiple cellular proteins, including paxillin and α- actinin, which control the function of cellular adhesion structures [75, 76]. These events induce a reorganization of actin-binding proteins in such specialized regions and have a considerable impact on cell contractility and subsequent motility. In addition, it has been reported that HBx is able to cause the rearrangement and the redistribution of other cellular adhesion molecules, such as CD44 [77]. This cell surface glycoprotein represents a major receptor for hyaluronan and it is also localized in the cellular pseudopodial tips and interacts with cytoskeleton by means of adaptor proteins, contributing to stabilize its architecture and, as a consequence, cellular form. Other transmembrane structures mediating normal cell-cell adhesions are represented by adherens junctions, desmosomes and tight junctions [78]. These complexes constitute the “epithelial junctional complex” and regulate epithelial cell polarization, differentiation and migration [78]. The most important transmembrane proteins of adherens junctions belong to the family called cadherins and E-cadherin is the best characterized member [79]. In epithelia E-cadherin binds β-catenin and this complex associates with F-actin and with adaptor proteins, including actinin-α and vinculin [80]. Anchorage of cadherin to cytoskeleton controls cellular shape and function. It has been reported that HBx may cause also the disruption of adherens junctions, by means of a Src-dependent phosphorylation of β-catenin, inducing the dissociation of E-cadherins, from cellular cytoskeleton [81]. Additional studies showed that HBx-carrying cells present decreased expression levels of wild-type β-catenin and E-cadherin, enhanced N-cadherin production, as well as reduced adhesion, in vitro, to fibronectin, an extracellular-matrix protein. Therefore, these events cause interference and perturbation of intercellular adherens junctions structure and produce changes in the architecture of cellular cytoskeleton [82, 83]. The latter event is characterized by a down-regulation of α5β1integrin in these cells and the redistribution of activated- β1integrin at pseudopodia tips induces an alteration of the balance existing between cellular adhesion/de-adhesion status [84]. Overall, the resulting HBx-modulated interaction with intercellular adherens junctions and perturbation of their structure produces changes in the architecture of cellular cytoskeleton. The consequent alteration of several cellular paths generally promotes a migratory phenotype of these cells and it may contribute to explain their acquisition of increased proliferative abilities as well as of invasive and metastatic properties [85]. Furthermore, HCV proteins also may interact with components of cytoskeleton, affecting its organization and, therefore, cellular shape and function. It has been demonstrated that polymerization of both microtubules and actin filaments is a necessary process for HCV-RNA synthesis [86]. In particular, HCV replication complexes associate directly with cellular microtubules and actin filaments, but not with intermediate filaments, both in HCV replicon, as well as in HCV-infected cells, and this interaction is mediated by two non-structural HCV proteins, including NS3 and NS5A [87]. Furthermore, a HCVmediated enhancement of FAK activity has been observed in a permissive hepatoma cell line [88]. As previously reported in some HBx-infected cellular lines, it has been observed that HCV-dependent FAK activation in human hepatoma and stellate cells is able to promote a direct α-actinin and paxillin phosphorylation, causing the displacement of α- actinin from plasma membrane to cytoplasm and an enhanced expression of paxillin [88]. However, it has to be considered that a direct interaction between α-actinin and HCV-NS5B protein has been also demonstrated [89]. Furthermore, it has been observed that, in a line of B-lymphoid leukemia cells, HCV-NS5A protein has established a direct link with a member of Src family kinase, in a tyrosine phosphorylation-mediated manner [90]. According to a recent research, some HCV structural components have been observed to perturb the localization of tight junction-bound proteins in Huh7 cells [91]. In particular, these adhesion complexes exert a key role in cell-cell interactions [92], modulating nuclear-junction interplay, functioning as a selective diffusion barrier and preventing the passage of certain soluble molecules [93]. Several integral membrane proteins are detectable in tight-junctions, such as: occludin, claudin-1 and zonula occludens protein-1. HCV envelope 2 glycoprotein is able to bind these cellular elements, inducing their retention in endoplasmic reticulum and de-arrangement of tight junction organization.
Figure 2. Intracellular sites of hepatitis B x protein (HBx) activity are shown. HBx may up-regulate the activity of 7 genes, which control the synthesis of some proteins involved in the organization of different cytoskeletal components: microtubule tubulin-β2, tubulin-β3 and tubulin-β6, periplakin, keratin 8, keratin 18 and actin-γ. Furthermore, HBx regulates the activity of several transcription factors, via modulation of cellular signalling transduction paths, such as Ras-Raf-MAP kinase, and Src family kinase cascades, it causes the rearrangements of some cellular adhesion molecules, such as CD44 and it facilitates the association of some adaptor proteins, such as Shc, growth factorreceptor- bound-2 (GRB-2) and son-of-sevenless (SOS). HBx also induces the activation of focal adhesion tyrosine-kinase (FAK), increasing the function of multiple cellular proteins, including paxillin and α-actinin. HBx may cause also the disruption of adherens junctions, by means of a Src-dependent phosphorylation of β-catenin, inducing the dissociation of Ecadherins, from cellular cytoskeleton as well as a decreased expression levels of wild-type β-catenin and E-cadherin. These events cause interference and perturbation of intercellular adherens junctions structure and produce changes in the architecture of cellular cytoskeleton.
Figure 3. Intracellular sites of NS3, NS5A and NS5B action are shown. Polymerization of both microtubules and actin filaments is a necessary process for HCV-RNA synthesis. In particular, HCV replication complexes associate directly with cellular microtubules and actin filaments, this interaction is modulated by NS3 and NS5A. These HCV related-proteins also induce the activation of focal adhesion tyrosine-kinase (FAK), increasing the function of multiple cellular proteins, including paxillin and α-actinin. However, NS5B may establish a direct interaction with α-actinin. HCV structural components have been also showed to perturb the localization of tight junction (T-J)-bound proteins in cell lines. These events cause interference and perturbation of intercellular adherens junctions’ structure and produce changes in the architecture of cellular cytoskeleton.
It has been observed that both HBV and HCV proteins may establish contacts with highly conserved domains of cellular components, via a direct interaction. In particular, common regions, defined as Src homology 2 and 3 (SH2 and SH3), are detectable in different signaling elements inside cells, including some members of Src family tyrosine-kinases and focal adhesion proteins, such as vinexin-β [94]. HBx and HCV NS5A are able to associate with these domains, up-regulating the function of these cellular components, therefore perturbing the activity of the Rho-family small GTPases.
The final result of these events is represented by the deregulation of cytoskeleton organization [95] and by increased mechanical stiffness both in liver [96] and pancreas [60]. Integrins play a critical role in this complex process. In particular, an enhanced expression of β1 and β6, as well as of α1, α5, α6, β1 and β6 integrin chains, has been reported in patients with HBV- and HCV-related chronic hepatitis, respectively and it has been associated both with liver necroinflammatory activity and with stage of fibrosis [96, 97, 98]. Furthermore, both viruses have been also observed to induce modifications of hepatic stellate cell functions. In particular, it has shown that HBV and HCV may infect and replicate in hepatic stellate cultured-cells, influencing gene expression of ECM-related molecules in these cells, such as collagen type I mRNA [99, 100], as well as a paracrine activation and proliferation of human hepatic stellate cell have been induced via use of conditioned cell-culture media, produced by HBx-, HCV-NS3- and HCV-NS5 expressing hepatocytes, by means of the TGF-β path [101, 102]. On the other hand, although recent studies have shown that HBV [103, 104] and HCV [105] are able to entry into hepatocytes, by interacting with conformational heparan sulfate binding sites on glycosaminoglycans side chains of cell-surface associated proteoglycans, to date, it is still unknown whether both viruses are able to modulate directly the synthesis of these glycoproteins and/or interfere with the process of their deposition in ECM.
Putative Mechanisms Involved in HBV- and HCVMediated Perturbation of Tissue “tensegrity” During Carcinogenesis
As described in previous sections of the present report, the process of HBV- and HCV-induced oncogenesis is characterized by a severe perturbation of several signaling pathways, which control important cellular functions, such as proliferation, differentiation, energy production and apoptosis, and it is mediated by some viral proteins. A large series of studies investigated and identified the interactive and dynamic interplay, which occurs during the process of carcinogenesis among liver and pancreatic cells, which progressively are acquiring a malignant phenotype, stromal cells and surrounding extracellular matrix. The association of viral proteins with components of cellular cytoskeleton causes the rearrangement and reconfiguration of its structure, via an increased activity of Rho kinases. On the other hand viral infection may induce a considerable alteration of extracellular matrix composition, influencing strongly its rigidity and stiffness. These events cause a direct perturbation of normal balance existing between intracellular and extracellular tensional forces, which are generated by cytoskeleton and extracellular matrix adhesions, respectively. Although the reported researches have identified a large number of viral components, which specifically interact with cellular targets such as some cytoskeletal elements, and suggested different mechanisms to explain the HBV- and HCVrelated carcinogenesis, not only in hepatic but also in pancreatic cells, to date no systematic model has been proposed to describe in a comprehensive manner how these pathogens may exert their oncogenic role. Starting from the concept that mechanical loads act as developmental regulators, several years ago Donald E Ingber proposed to apply the “tensional integrity” or “tensegrity model” to biological systems. Cell shape strongly influences crucial cellular activities, including biochemistry and gene expression. Taking advantage from this large number of available studies, we now propose a unifying hypothesis that allows to correlate HBV and HCV infection, “tensional integrity” model, changes in cellular functions and carcinogenesis. According to our assumption, both viruses induce a progressive perturbation of cellular and extracellular microenvironment, generating a critical modification of cytoskeleton, as well as of outer matrix stiffness. Integrins and cadherins sense the changing gradients of tissue rigidity, which is progressively caused by viral infection. This event perturbs the tightly-regulated feedback loop, which usually couples the intracellulargenerated forces to substrate rigidity of extracellular compartments. Therefore, such a change strongly affects intracellular functions, promoting a substantial deregulation of critical intracellular biochemical activities, as well as of genome expression and, as a consequence, influencing cellular fate. The study of stiffness in human organs is not only an experimental concept without direct consequences, but the assessment of this parameter is progressively acquiring a pivotal role in clinical practice. In the last years, noninvasive tests for assessment of fibrosis and liver stiffness have been used in patients with chronic HBV and HCV infections. It has been reported that the risk of hepatocellular carcinoma and/or mortality development is significantly higher in patients with HBV/HCV-related cirrhosis and elevated levels of liver stiffness [106, 107].
Our hypothesis might provide for the first time a reliable system, which correlates tensional integrity model with intra- and extra-cellular modifications, occurring in liver and pancreas during HBV- and HCV-induced carcinogenesis. This approach might contribute to increase our knowledge of pathogenetic mechanisms involved in the development of several pancreatic and hepatic diseases, as well as to improve the possibility of their treatment. Furthermore, the usefulness of this model should be definitively confirmed and it might be also helpful to extend its field of application to other viruses-related cancers. The immediate consequence of this novel approach might have a substantial impact on diagnosis, prevention, treatment and overall management of several malignancies, whose development has been correlated with the presence of an infection caused by oncogenic viruses.
The authors thank Dr. Simonetta Righi, Central Library, S. Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy for her support in the search of scientific bibliography
None declared