Quality Improvement Report - (2011) Volume 19, Issue 6
Research Associate, National Travel Health Network and Centre, University College London Hospitals, London, UK
Hilary Simons RGN RSCN Dip N MSc FFTM (RCPS Glasg)
Senior Nurse, National Travel Health Network and Centre, University College London Hospitals, London, UK and Liverpool School of Tropical Medicine, Liverpool, UK
Naomi Launders BSc MSc
Lead, Verotoxin-producing Escherichia coli, Health Protection Agency, Health Protection Services, Colindale, UK
David R Hill MD DTMH FRCP FFTM (RCPS Glasg)*
Director, National Travel Health Network and Centre, University College London Hospitals, London, UK and Honorary Professor, London School of Hygiene and Tropical Medicine, London, UK
Received date: 20 May 2011; Accepted date: 3 October 2011
Background The National Travel Health Network and Centre (NaTHNaC), a United Kingdom public health body, is responsible for designating nearly 3500 Yellow Fever Vaccination Centres (YFVCs) in England, Wales and Northern Ireland (EWNI). In 2005, NaTHNaC established a programme of registration, training, clinical standards and audit for YFVCs following the mandate of International Health Regulations (IHR, 2005). Assessment of problemAdministration of yellow fever (YF) vaccine is complex because of the changing epidemiology of YF and the risk of rare, severe adverse events following vaccination. Additionally, there is little formal assessment of providers of travel medicine, particularly in the area of YF vaccination. In 2004, prior to introducing their programme, NaTHNaC sent a questionnaire to allYFVCs in England to assess their practice. This highlighted a need for training and institution of standards to reinforce best practice in vaccination and knowledge about YF. Strategies for changeIn 2005, NaTHNaC introduced its programme for all YFVCs. It was expected that training, adherence to standards and access to resources would lead to increased confidence and consistency of practice by YF vaccine providers. Effects of changeIn 2009, a questionnaire was sent to all YFVCs in EWNI to evaluate the impact of the NaTHNaC programme. Among respondents who attended NaTHNaC training 95.8% of respondents indicated that it improved their confidence about YF vaccination. Furthermore, 68.5% of centres made changes to their practice, and improved adherence to core standards was observed. Next steps and lessons learned The NaTHNaC programme has led to improved standards in YFVCs and increased confidence in health professionals who administer the YF vaccine. Although this has not been tested, it is expected that this will translate to more consistent and better care for the international traveller. Elements of the NaTHNaC programme could be a model for improvement of clinical standards and for other countries as they seek to implement IHR (2005) and improve the practice of travel medicine.
audit and feedback, general practice, primary care, quality improvement, training, vac-cination, yellow fever
Yellow fever (YF) is an acute viral haemorrhagic fever endemic in many tropical areas of South America and Africa.[1,2] The control of YF has been through vacci-nation of individuals at risk and control of the mos-quito vectors, particularly Aedes spp. in urban areas of endemic countries. Vaccination against YF for inter-national travel is regulated under International Health Regulations (IHR 2005).[3] In 2005, IHR were revised with the goals of improving the surveillance, identifi-cation, response and notification of public health emergencies of international concern. As part of the application of prevention measures at international ports, YF vaccination can be required by individual countries as a condition of entry, with the primary goal of preventing the introduction of YF.[3] The YF vaccine is also administered to protect travellers at risk. YF is currently the only disease for which an International Certificate of Vaccination or Prophy-laxis (ICVP) may be required for entry into a country.[4]
In the United Kingdom (UK), administration of the YF vaccine is undertaken by specifically designated Yellow Fever Vaccination Centres (YFVCs). One of the key remits of the National Travel Health Network and Centre (NaTHNaC), when it was established in 2002 by the Department of Health (England), was the responsibility for designating YFVCs in England, and subsequently in Wales and Northern Ireland (EWNI). With approximately 3500 YFVCs representing nearly a third of all practices in EWNI, it is a priority that these centres practice to an agreed standard.
NaTHNaC is a public health body, commissioned by the Health Protection Agency (HPA), with the broad goal of Protecting the Health of British Travellers by helping to set standards in travel medicine. NaTHNaC’s key objectives are to: provide timely, evidence-based advice on travel medicine practice and global health events that might affect British travellers; contribute to surveillance of imported in-fections; administer YFVCs; engage stakeholders in travel medicine; provide education and training op-portunities; and contribute to setting research pri-orities. To this end, NaTHNaC provides an open access website (www.NaTHNaC.org), runs a national telephone advice line and has published the definitive text resource for travel medicine in the UK.[5]
There is little formal assessment of the knowledge and competency of providers of travel medicine in general practice. As such, NaTHNaC is interested in eval-uating the impact of its programme of registration, training, clinical standards and audit on the clinical practice of travel medicine in YFVCs.
YF vaccination is complex due to changes in country requirements for vaccination, the epidemiology of YF and the potential for severe and potentially life-threat-ening adverse events following vaccination.[2,6–10] In addition, there are increasing numbers of travellers with special health needs going to areas at risk of YF transmission. Of the 5.8 million UK residents who travelled overseas in 2009, it is estimated that 820 000 went to YF-risk countries.[11] These issues necessitate YFVCs carrying out an accurate risk assessment that balances the traveller’s itinerary and health status, with the safety of YF vaccine. The overall goal of NaTHNaC’s programme is to improve the standard of care around YF vaccination and ultimately to improve the practice of travel medicine.[4]
In 2004, prior to implementing their programme, NaTHNaC sent a questionnaire to all YFVCs in England to assess their practice and perceived needs.[12] The questionnaire was designed following a review of the literature on best practice in travel medicine, and piloted with travel medicine nurses. It covered: type of practice, administration of travel vaccines, training and duties of staff, vaccine storage and record keeping, access to travel health information, and resource and training needs. The YFVCs were identified by a database held by the Department of Health that listed 4385 YFVCs in England.
A total of 2933 questionnaires were completed, achieving a 69.1% response rate. It is highly likely that the centres that did not respond were no longer practising as YFVCs, since each centre was required to update their details if they wished to continue to administer the YF vaccine. The following were key Results:[12]
• 94% of YFVCs were in National Health Service (NHS) general practice (GP) settings
flat least 32% of GP surgeries in England were giving the YF vaccine
• relatively few doses of YF vaccine (median 35) were administered annually by each centre
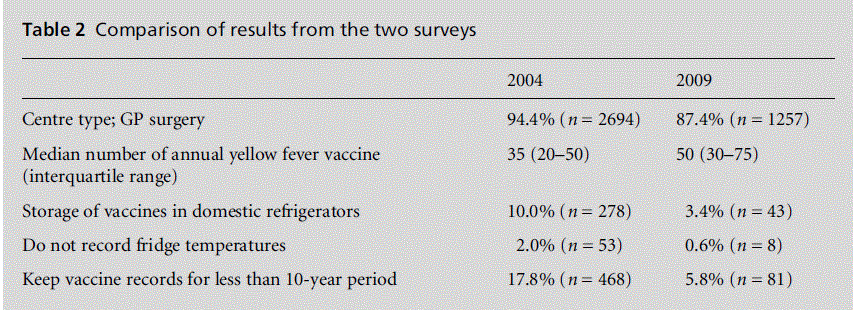
• 10% of centres stored vaccines in domestic refriger-ators; 2% did not record refrigerator temperatures, while nearly 16% of centres did not maintain vaccine records for the required 10-year period
• 95% of nurses working in YFVCs had received general training in travel medicine, however, fewer than 60% of physicians who were in charge of the centres had received such training (P < 0.0001). Only 30% of health professionals had received training in YF vaccination. Travel medicine training was most often delivered or sponsored by pharma-ceutical companies.
These findings highlighted a need for training to reinforce best practice in vaccination and knowledge about YF, which is delivered without potential com-mercial bias. It supported the intent by NaTHNaC to institute its programme of registration, training, stan-dards and audit.
The call for improved training and standards of YFVCs has been made by the World Health Organ-ization (WHO) in IHR (2005),[3] by the United States Centres for Disease Control and Prevention,[13] and in the literature.[4,14–16] Although the dominant model of delivery of travel medicine within primary care is convenient to the traveller, the low number of annual YF vaccinations (fewer than one per week) also raised the question of whether patients, who were being seen at practices administering very few doses, were receiv-ing an appropriate level of care.
In 2005, NaTHNaC established a programme of regis-tration, training, clinical standards and audit for YFVCs following the mandate of IHR (2005): ‘State parties shall designate specific yellow fever vaccination centres within their territories in order to assure the quality and safety of the procedures and materials employed’.[3] The legislative authority for NaTHNaC to do this on behalf of the Department of Health and HPA falls under the Health Protection Agency Act 2004 and Regulation 7(a) of The Health Protection Agency Regulations 2005.[17] In July 2005, NaTHNaC’s responsibility was extended to Wales by the direction of the Welsh Assembly[18] and in October 2007 to Northern Ireland by Direction of the Department of Health, Social Services and Public Safety, Belfast.[19] Health Protection Scotland has a similar programme for YFVCs in Scotland based on the NaTHNaC model.
After rolling out the programme, all YFVCs became bound by the obligations of the Code of Practice (www. NaTHNaC.org/pro/YFvcinitiative.htm#appendix), and centres had to update their practice to comply. Some of the standards include:
• Staff must be trained to advise travellers when the YF vaccine is recommended and/or required, and be competent in the safe administration of YF vaccine. As such, a clinical member of each YFVC will attend an NaTHNaC-sponsored training session before designation status is granted, and thereafter every two years. Initial training comprises a full day, with renewal training delivered over half a day. NaTHNaC staff or NaTHNaC-trained travel medi-cine experts deliver all training.
• Facilities for administering and storing vaccines will conform to acceptable standards.
flappropriate records for all vaccinations must be maintained for 10 years.
NaTHNaC also established an agreement with SanofiPasteur MSD, the manufacturer and supplier of the YF vaccine in the UK, that SanofiPasteur MSD would only provide vaccine to centres that had been desig-nated by NaTHNaC. Thus, an NHS, private, occu-pational health or military practice in EWNI cannot administer the YF vaccine without first applying to NaTHNaC for designation status and undergoing training.
In 2009, a follow-up questionnaire was sent to all YFVCs in EWNI to evaluate the impact of the NaTHNaC programme (see supplementary informa-tion online at www.radcliffe-oxford.com/journals/J10Quality_in_Primary_Care/Supplementary_Papers.htm).[20] As part of the Code of Practice, YFVCs agree to have their practice audited. Key measures of improvement from the perspective of travellers receiving the YF vaccine are: ready access to YFVCs; the assurance that providers are knowledgeable in the assessment of the YF geographical risk and requirements under IHR (2005); that providers administer vaccine safely, and that there is consistency of advice between YFVCs. Clinical care by YFVCs will be improved by compli-ance with standards (e.g. record keeping and vaccine storage), having access to evidence-based resources for making a YF risk assessment (including on-line resources and a national telephone advice line) and a regular programme of training around YF vacci-nation. This should lead to increased confidence of clinicians about complex YF issues.
Of the 3465 YFVCs in EWNI that were registered when the 2009 questionnaire was sent, a total of 1462 centres responded, and 1438 centres completed the entire survey (41.5%).[20] Responses were reviewed by geo-graphical area (postcode) with 71.6% of postcode areas having a response rate of between 31 and 50%. Response rates to individual questions ranged from 72.6 to 99.9%. The majority of respondents were from GP practices (87.4%); occupational health centres (4.0%), private travel clinics (3.5%), private health facilities (2.4%) and other types of practice (2.7%) comprised the remainder of respondents. Those com-pleting the questionnaire were most often the practice nurse (43.0%) or the nurse responsible for the YFVC (41.8%). The majority (76.6%) of centres had become a YFVC before January 2005, when the NaTHNaC programme was implemented.
A median of 50 doses (interquartile range (IQR), 30– 75 doses) of YF vaccine were given annually by YFVCs. There was a significant difference in the number of doses given by clinic type (P < 0.005), with private travel clinics administering more doses than other settings.
A total of 1326 respondents (92.7%) stated that they had received either full- or half-day training from NaTHNaC. Nearly all (95.8%) indicated that the NaTHNaC training improved their confidence on YF vaccine issues. Initial training is provided by core nursing staff of NaTHNaC in a full-day session, and update training (every two years) by specifically com-missioned and trained UK travel medicine experts in a half-day session. The content includes didactic lec-tures and interactive case-based scenarios. The lec-tures cover the role of NaTHNaC in travel health, YF disease and epidemiology, YF vaccine and safety, and how YF vaccination fits into the NaTHNaC Code of Practice and IHR (2005). The clinical scenarios are taken from the NaTHNaC telephone advice line, and allow attendees to discuss and debate complex issues relating to YF vaccination. Attendees also complete a pre- and post-training test that provides them with a benchmark of their understanding of key issues.
After training, 68.5% (890/1300 respondents) of centres made changes to their practice: in risk assess-ment for YF vaccination (61.9%), record keeping (61.6%) and use of internet resources for YF informa-tion (48.1%). GP surgeries were most likely to make changes compared with other centre types (P < 0.005; Table 1), however, the size of the YFVC (based on number of travel medicine patients seen annually) did not affect whether practice changes were made.
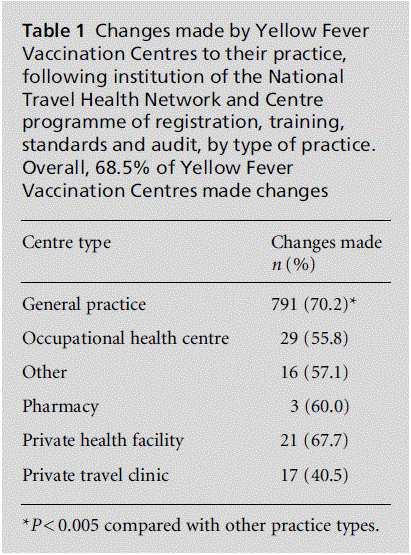
There was improved adherence to the core stan-dards in comparison with the baseline study (Table 2).[12] Only 3.4% (n = 43) of YFVCs stored vaccines in a domestic refrigerator (with no internal/external ther-mometer) compared with 10% in the baseline study (P < 0.001 by Kruskal–Wallis test), 0.6% (n = 8) did not record refrigerator temperatures (compared with 2%, P < 0.001) and only 5.8% (n = 81) of centres kept vaccine records for less than the required 10-year period (compared with 15.7%, P < 0.001).
Following training, the proportion of practitioners who felt highly confident about YF vaccination ranged from 76.4 to 97.8% (Figure 1). Respondents were highly confident about the storage (97.8%) and admin-istration (96.8%) of YF vaccine, but less so about making risk assessments for those with chronic medi-cal conditions and who were age 60 years and older.
Figure 1: Respondents with a high level of confidence about YF vaccination following attendance atNaTHNaC yellow fever training course (n = 1326), compared with those who had not attended training (n = 104). Those who received training were more likely to have high confidence levels in all categories, P < 0.001. High confidence was indicated by a self-selected confidence score of 4 or 5, on a 5-point scale. NaTHNaC, National Travel Health Network and Centre; YF, yellow fever; YFV, yellow fever vaccine; IHR, International Health Regulations
A difference in confidence levels was apparent between those who did and did not attend training (Figure 1). Of those not attending training, only 56.4% expressed confidence about the global epidemi-ology and transmission of YF compared with 84.3% of those who had been trained (P < 0.001). Among those without training, 58% expressed high confidence about IHR (2005) compared with 80.2% of those who had received training (P < 0.001). The main reasons for the lower confidence among those who had not received training were their lack of training (55.6%) and experience (44.4%).
This follow-up survey of YFVCs enabled us to evaluate the impact of the NaTHNaC programme of regis-tration, training, standards and audit on clinical prac-tice by YFVCs in EWNI. The programme has been associated with improved adherence to basic stan-dards of immunisation practice and with high confi-dence levels of healthcare providers in YF vaccination. It is expected that improved standards and increased confidence will improve quality through consistent and better care for international travellers who visit GP surgeries or private travel medicine clinics. How-ever, this has not been formally tested in the field of travel medicine, and the impact of clinical guidelines in general practice has been mixed.[21] The establish-ment of a nationally approved programme that re-quires YFVCs to register with NaTHNaC, attend training and sign a Code of Practice outlining clear practice standards, are likely to be key features that lead to practice improvements. In addition, YFVCs are not able to buy YF vaccine if they have not undergone the designation process. NaTHNaC also frequently communicates with YFVCs via email alerts and news-letters, answers their clinical queries on our national advice line and posts updated information on our website. These are all measures that can successfully lead to practice change; the first step in trying to improve care and outcomes.[22]
In order to determine whether standards are main-tained, NaTHNaC has developed an assessment and audit instrument that will audit vaccine storage, record keeping and administration. It will also assess responses to clinical scenarios that will be answered by the healthcare providers at the YFVCs. NaTHNaC also plans to explore whether the number of YF vaccine doses given annually correlates with accuracy in answer-ing the clinical scenarios. There will be specific feed-back to centres based on their performance on the audit; this audit will be rolled out in late 2011.
The study has also helped to identify areas of relatively low confidence where the NaTHNaC train-ing can be modified. These are in the areas of YF vaccine indications for persons who are elderly or with special health needs, the epidemiology of YF risk and how IHR (2005) apply to YF vaccination (Figure 1). YFVCs have responsibility for making appropriate decisions when these complex situations arise in prac-tice, however, they do have the option to discuss them with NaTHNaC over their advice line.
In order to allow more clinical personnel in each YFVC to have access to training, NaTHNaC will develop on-line training for YFVCs that are renewing their registration. Continuing professional develop-ment credits have been provided for all NaTHNaC YF training to provide added value. These efforts should help to overcome both lack of confidence and experi-ence, and will be important to some practices, that based on the estimated number of YF vaccine doses given, have only limited opportunities to see patients travelling to YF risk countries.
The major limitation of the study was that the response rate was lower than in the baseline study. It is not possible to know whether the YFVCs that felt they had benefitted from the NaTHNaC programme were more or less likely to complete the questionnaire than those centres that did not consider the pro-gramme to be beneficial. However, the distribution of the YFVCs that completed the questionnaire was similar to the complete database in terms of location. The response rate was likely to be affected by the demands placed upon healthcare providers during the influenza pandemic of 2009. It is also possible that factors, such as other training opportunities or greater awareness of immunisation standards, could have led to improvements in practice.
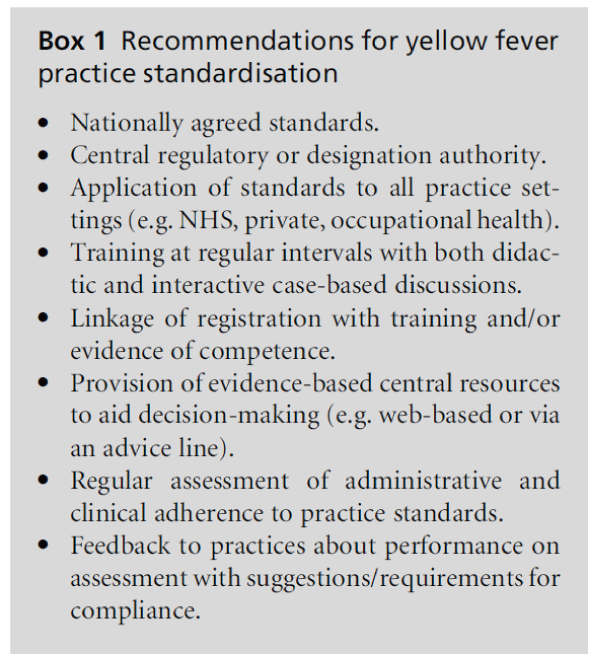
The lessons learned from implementation of a standard for YF vaccination could be applied to other areas of clinical practice (Box 1).
Internationally, although several WHO Member States have developed a process of designation of specific YFVCs in accordance with the IHR (2005), only rarely has it been tied to standards, education and audit.[16,23–25] The successes seen with the NaTHNaC programme could be a model for other countries as they seek to implement IHR (2005) and improve the quality of practice of travel medicine.
NaTHNaC thanks the dedicated travel medicine pro-fessionals who deliver NaTHNaC’s training to YFVCs (Lynda Bramham, Norma Evans, Mary Gawthrop, Hilary Simons, Alexandra Stillwell, Claire Wong, Alison Jenkins, Carolyn Driver, Cate Riley, Joanna Lowry, Kath Lynch, Sandra Grieve, Sarah Lang, Shirley Bannatyne and Dr Steve Riley). We also thank the administration staff who provide the logistical support for the YFVC programme (Stella Bailey, Geraldine Oliver, John Mathewson and Yetunde Ibitoye).
HS and DRH conceived the questionnaire. Each author contributed to its design and implementation. NB posted the questionnaire on-line and collected all of the data. NB sorted and cleaned the data, and NB and NL analysed it. NB wrote the first draft of the paper. DRH extensively revised the draft, and submit-ted a revised manuscript for HS and NL to review. HS and NL made comments and changes to the manu-script that were reviewed by NB and DRH. DRH is the guarantor of the final submission.
No external source of Funding. Study performed with the National Travel Health Network and Centre budget.
Not applicable.
Not commissioned, externally Peer Reviewed.
None. NaTHNaC charges a registration fee from YFVCs that goes to Funding the programme and to the general operating budget of NaTHNaC.