Case Series - (2017) Volume 18, Issue 4
1Department of Radiology, Aichi Medical University, 1-1 Yazako Karimata, Nagakute, 480-1195, Japan
2Department of Radiology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showaku, Nagoya, 466-8550, Japan
3Division of Surgical Oncology, Department of Surgery, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, 466-8550, Japan
Received April 28th, 2017-Accepted May 10th, 2017
Objective Postoperative bleeding is a severe complication that can occur after pancreatectomy. Bleeding mainly arises from peripancreatic arteries, but the portal venous system can also rarely cause bleeding. This study assessed portal vein bleeding after pancreatectomy. Methods Between November 2013 and August 2016, five patients (three males, two females; median age, 75 years; age range, 66-78 years) with postoperative portal vein bleeding underwent stent graft repair. Angiography and fistulography were performed in four and five patients, respectively. Patients were retrospectively analyzed with regard to radiological findings and clinical outcome. Results All patients developed pancreatic leakage after surgery. Angiography demonstrated portal vein pseudoaneurysm in one patient and no evidence of bleeding in the other three patients. Fistulography demonstrated the peritoneal cavity with pancreatic leakage connecting with the portal venous system in all five patients. Stent grafts achieved hemostasis with portal flow preservation in all patients. Four patients were discharged and one patient died of multiorgan failure in hospital. Conclusions Fistulography is useful to depict sources of portal vein bleeding. Stent grafts appear to offer effective treatment for portal vein bleeding after pancreatectomy.
Aneurysm, False; Pancreas; Pancreatectomy; Portal vein
Despite declining mortality rates after pancreatic surgery, postoperative bleeding remains one of the most serious complications, particularly in high-volume centers [1, 2, 3]. Bleeding occurs in 1-8% of cases, but accounts for 11-38% of mortality [4, 5, 6]. The peripancreatic vascular structures that may be sources of bleeding are mainly the stump of the gastroduodenal artery, hepatic artery, splenic artery, or a branch of the superior mesenteric artery [1, 3, 4, 5, 6]. Although quite a rare occurrence, the portal venous system can also be a source of postoperative bleeding. The frequency of portal vein bleeding after pancreatectomy is reportedly <1% (0.1-0.7%) [7, 8, 9]. Like arterial bleeding, portal vein bleeding also can cause morbidity [7].
With recent advances in interventional procedures, embolization and stent grafts have been widely used, gaining acceptance for the treatment of arterial bleeding after pancreatectomy. Systematic reviews comparing endovascular therapy and laparotomy for postpancreatectomy bleeding have revealed that hemostasis stays about the same (endovascular therapy, 76-80%; laparotomy, 73-76%). However, the mortality rate associated with endovascular therapy appears lower than that with laparotomy (20-22% vs. 43-47%, respectively) [10, 11].
As to bleeding from the portal vein, no case series appear to have been reported. We describe herein the five consecutive patients with intra-abdominal bleeding from the portal vein after pancreatectomy in which stent graft repair was performed.
Patients
Our institutional review board approved the retrospective collection of data and data analysis for this study, and the requirement for informed consent from the patients was waived. Between November 2013 and August 2016, five consecutive patients (three males, two females; median age, 75 years; age range, 66-78 years) with portal vein bleeding after pancreatic surgery underwent placement of a stent graft at the portal vein. The primary tumor was bile duct carcinoma in two patients, and gall bladder carcinoma, ampullary carcinoma, and metastatic pancreatic tumor from renal cell carcinoma in one patient each.
The resection procedure for the tumor was pancreaticoduodenectomy in three patients, extrahepatic bile duct resection and partial resection of the pancreatic head in one patient, and distal pancreatectomy in one patient. Two patients also underwent right hepatectomy and caudate lobectomy combined with resection and reconstruction of the portal vein.
Stent Graft Procedures
Placement of a stent graft was performed as follows. In the first patient, definitive surgical repair for portal vein bleeding was attempted at the start. However, surgical repair was difficult because of severe postoperative intraabdominal adhesions. The therapeutic strategy was thus changed intraoperatively to stent graft deployment. The portal venous system was accessed by an ultrasoundguided transhepatic approach. An 18-gauge Cliny ultrasound puncture needle (Create Medic, Yokohama, Japan) was placed into a peripheral portal vein branch of the left lateral segment of the liver. The Seldinger technique was used to place a 5-Fr sheath into the main portal vein. Direct portography was then performed using a 4-Fr catheter. The diameter and length of the portal vein and the superior mesenteric vein were measured. The 5-Fr sheath was exchanged for a 12-Fr sheath over a guidewire. Then, a stent graft was deployed from the superior mesenteric vein to the main portal vein. Post-dilatation was performed with a balloon catheter. At the end of the procedure, the punctured hepatic tract was closed with 0.035-inch coils.
In the remaining four patients, stent graft repair was planned from the start. The portal venous system was accessed by a transileocolic approach. The ileocolic vein was punctured with an 18-gauge needle under laparotomy. And, a 12-Fr sheath (n=2), 14-Fr sheath (n=1), or 10-Fr sheath (n=1) was inserted to the superior mesenteric vein over a guidewire. Direct portography was then performed using a 4-Fr catheter, and the diameter and length of the portal vein and superior mesenteric vein were measured. A stent graft was then deployed. Postdilatation was performed with a balloon catheter.
The stent graft was selected based on the diameters of the portal vein and superior mesenteric vein. A Gore Excluder contralateral leg endoprosthesis (W. L. Gore & Associates, Flagstaff, AZ) was used in four patients, and a Fluency stent graft (Bard, Tempe, AZ) was used in one patient.
In two patients, the bleeding site was near the confluence of the splenic vein and superior mesenteric vein. The splenic vein and inferior mesenteric vein were thus embolized with 0.035-inch coils and/or Amplatzer vascular plug (St Jude Medical, St Paul, MN) before stent graft deployment.
Assessments
Patients were analyzed with regard to clinical findings, diagnosis of portal vein bleeding, stent graft deployment, and clinical outcome. To clarify the effects of stent graft repair, results were assessed with regard to technical success and clinical efficacy. Technical success was defined as exact deployment of the stent graft to cover the site of portal venous injury without complication, and preservation of portal flow at final angiography. Clinical efficacy was defined as freedom from portal vein bleeding after stent graft deployment.
Clinical and Radiological Findings
Clinical and radiological findings are summarized in Table 1. All five patients developed pancreatic leakage after pancreatectomy. According to the International Study Group on Pancreatic Fistula grading [12], four patients suffered grade B pancreatic leakage and one patient suffered grade C pancreatic leakage.
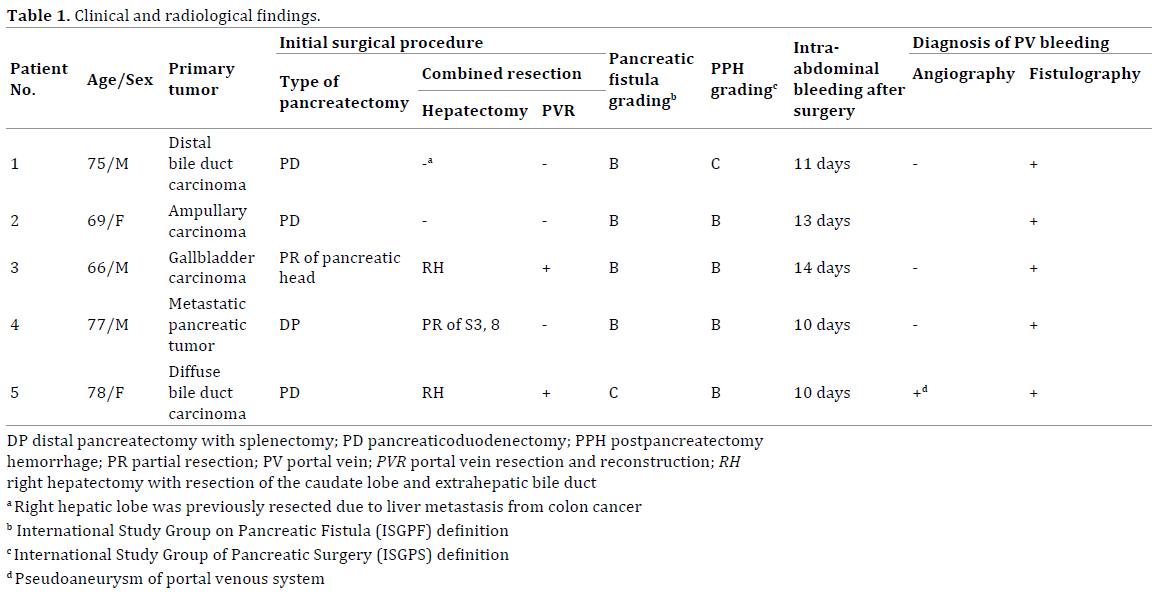
Intra-abdominal bleeding through the intraoperatively placed drains was identified 10-14 days (median, 11 days) after initial surgery. All patients were managed by transfusion of red blood cell and/or intravenous fluids. According to the International Study Group of Pancreatic Surgery grading [13], four patients suffered grade B bleeding and one patient suffered grade C bleeding.
Emergency angiography was performed as the initial management tool for intra-abdominal bleeding in four patients. Selective angiography of the celiac, common hepatic, and superior mesenteric artery demonstrated no evidence of arterial bleeding. Venous-phase selective angiography was also acquired as indirect portography. Indirect portography demonstrated no evidence of venous leakage. However, one patient showed pseudoaneurysm at the splenic vein near the confluence with the superior mesenteric vein (Figure 1a, b).
Figure 1. Patient No. 5: (a). Indirect portography after selective celiac arteriography and (b). 3-dimensional CT angiography show pseudoaneurysm of the splenic vein near the confluence (arrow). (c). Portography after stent graft deployment (arrowhead) shows good portal flow. The splenic vein (white arrow) and inferior mesenteric vein (black arrow) are embolized with a vascular plug and coils, respectively.
Following angiography to reposition and/or exchange the peripancreatic drain, traction was placed on the drains and contrast medium was injected via the drains in four patients. In three of these four patients, this contrast study showed communication between the peritoneal cavity and portal venous system, indicating portal vein bleeding (Figure 2a, b). In the other patient, no bleeding site was identified at that time and tentative bleeding repeated during follow-up. At 15 days after first bleeding, fistulography showed the portal vein and re-bleeding was present. In the remaining patient without angiography, bleeding occurred during fistulography, and the portal vein was demonstrated on fistulography. Finally, portal vein bleeding was diagnosed by fistulography in all patients. After fistulography, no patients developed severe infections such as bacteremia.
Figure 2. Patient No. 3: (a). Fistulography from the drain shows the cavity of the pancreatic leakage that communicates with the portal venous system. (b). Direct portography from the superior mesenteric vein shows contrast extravasation into the tract of the drain. (c). Portography after stent graft deployment shows no contrast extravasation and good portal flow.
The bleeding site of the portal venous system was the main portal vein in two patients, the confluence of the superior mesenteric vein and portal vein in one patient, the stump of the splenic vein in one patient, and the splenic vein near the confluence in one patient. In four patients, the bleeding site was where the operatively placed peripancreatic drain crossed on both fistulography and computed tomography (CT) scan. In one patient with portal vein pseudoaneurysm, the bleeding site was separate from the drain, but was enclosed by pancreatic leakage.
Stent Graft Repair
The results of stent graft repair are summarized in Table 2. The interval between primary surgery and stent graft deployment was 10-25 days (median, 13 days). In all patients, stent grafts were successfully deployed to cover the site of portal venous injury. And, final portography after stent graft deployment showed good portal flow (Figure 1c, 2c). Thus, clinical efficacy was achieved in all five patients.

Clinical Course
Clinical courses after stent graft placement are summarized in Table 3. The follow-up period was 2-23 months (median, 9 months). After the procedure, the patients remained free of further portal vein bleeding. Thus, clinical efficacy was achieved in all five patients. Four patients were discharge in good health, but the remaining patient died of multiorgan failure two months after placement of a stent graft.
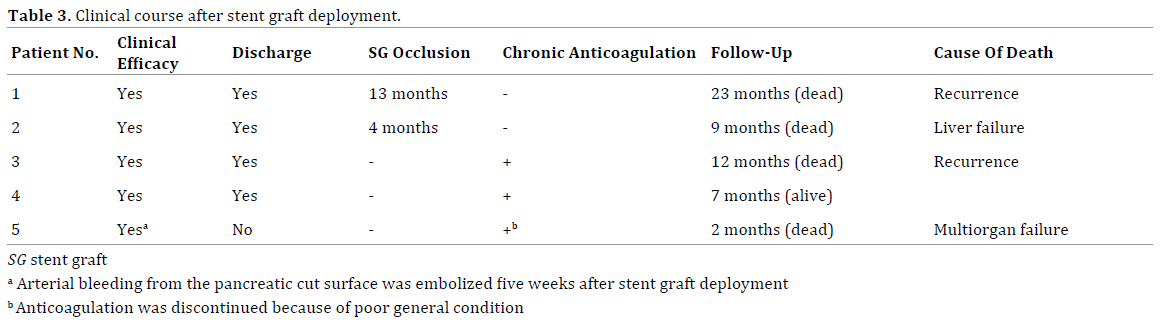
During follow-up, patency of the stent graft was assessed by Doppler ultrasonography and/or contrastenhanced CT scan every three-six months. The stent graft was occluded with thrombus after 13 months in the first patient, and after four months in the second patient. The two earliest patients had not been receiving chronic anticoagulation therapy. Systemic anticoagulation therapy was initiated after stent graft occlusion. However, recanalization of portal flow was not achieved. The last three patients therefore received chronic anticoagulation therapy using warfarin after placement of a stent graft. However, anticoagulation therapy was immediately discontinued in one of the three patients because of poor general condition. All three patients remained free of stent graft thrombus during follow-up.
Although the exact mechanisms underlying postpancreatectomy bleeding remain elusive, pancreatic leakage and localized infection are thought to represent major contributors [5, 14]. The effects of localized infection and pancreatic juices likely weaken and erode the blood vessel walls. Mechanical irritation by the drain is another cause of bleeding [15]. In our series, pancreatic leakage occurred in all patients, and the drain was in contact with the bleeding site in four of the five patients. Previous articles of portal vein bleeding have also reported bleeding sites in proximity to the drain [16, 17].
Indirect portography following selective arteriography could not detect a bleeding source in three of the four patients. Previous article has also reported postulated causes of false-negative angiography, including pauses in intermittent bleeding or a venous origin of bleeding, which is difficult to identify after arteriography [2]. In one patient, indirect portography could demonstrate pseudoaneurysm at the splenic vein. Burke et al. [17] reported a similar case in which portal vein pseudoaneurysm was diagnosed by indirect portography. When arteriography fails to identify a bleeding source, consideration of the possibility of bleeding from the portal venous system is also important.
On the other hand, fistulography was helpful to identify bleeding sites involving the portal venous system in all five patients. Fistulography demonstrated not only a cavity associated with leakage, but also the portal vein, indicating portal vein bleeding. Yoshida et al. [8] and Ginsburg et al. [16] also described the utility of fistulography to diagnose portal vein bleeding. Our study agrees with previous reports, and fistulography seems to offer the valuable modality to elucidate bleeding sites of the portal vein.
Choice of treatment for portal vein bleeding after pancreatectomy may depend on the clinical presentation. Reported approaches to treatment include surgical repair [9, 17], stent grafting [16], and temporary balloon occlusion and conservation [8]. In our first patient, we abandoned definitive surgical repair because of severe intra-abdominal adhesions. The treatment strategy was therefore changed to stent graft repair intraoperatively. Our experience with this patient led us to select stent grafts as the first-line treatment in the next four patients.
The use of stent grafts in the treatment of postoperative arterial bleeding appears feasible, effective, and safe [18, 19]. Stent grafts are now considered the first-line treatment for arterial bleeding under critical condition in which the consequences of hepatic artery or superior mesenteric artery occlusion may be disastrous. In the same manner as for arterial bleeding, the use of stent grafts for portal vein bleeding offers a reliable treatment option. In our series, all patients achieved hemostasis and avoided rebleeding from the portal vein. However, surgery remains a therapeutic option when endovascular procedures are not available.
To deploy a stent graft at the portal vein, either a transhepatic or a transileocolic approach can be applied. Other reports [16, 20, 21] have selected the percutaneous transhepatic approach. The merit of that approach is the reduced invasiveness compared to the transileocolic approach, because the transileocolic approach requires laparotomy. However, a transileocolic approach also has some advantages in patients with postsurgical bleeding. Laparotomy allows the treatment of factors that contribute to bleeding, such as anastomotic disruption or intraabdominal collections.
The necessity of anticoagulation therapy after stent graft deployment at the portal vein remains unclear. Our first two patients were followed without chronic anticoagulation therapy, but the stent grafts were thrombosed after 13 months and 4 months, respectively. Later patients were therefore administered warfarin just in case. Although the follow-up periods were very short, the patency of the stent grafts has been preserved without thrombus. Further studies are required to evaluate the necessity of prophylactic anticoagulation therapy following stent graft deployment.
The present study showed limitations related to the retrospective design and small cohort size. This is because portal vein bleeding after pancreatectomy is quite a rare complication compared with arterial bleeding. Accumulation of further cases is thus necessary to assess the clinical features and efficacy of stent graft repair.
In conclusion, the portal vein has the potential to cause intra-abdominal bleeding after pancreatic surgery. Fistulography appears useful in diagnosing portal vein bleeding, and placement of a stent graft is available as a therapeutic option for portal vein bleeding after pancreatectomy.
The authors declare that they have no conflict of interest.