Original Article - (2017) Volume 18, Issue 6
Sia Kim1, Malinda Itchins2,3, Jennifer Arena2, Chris Nahm1,3, Nick Pavlakis2,3, Stephen Clarke2,3, Anthony Gill3,4, Jaswinder S Samra1,3, Anubhav Mittal1,3
1Upper Gastrointestinal Surgical Unit, Royal North Shore Hospital, Sydney NSW, Australia
2Department of Medical Oncology, Royal North Shore Hospital, Sydney NSW, Australia
3Northern Clinical School, Sydney Medical School, University of Sydney, NSW, Australia
4Cancer Diagnosis and Pathology, Kolling Institute, University of Sydney, NSW, Australia
Received Date: August 08th, 2017; Accepted Date: October 05th, 2017
Background Pancreatic ductal adenocarcinoma frequently recurs despite curative surgical resection. This study aims to investigate the patterns of recurrence following surgical resection of pancreatic ductal adenocarcinoma. By understanding the patterns of postoperative recurrence, more focused management strategies for advanced disease in pancreatic ductal adenocarcinoma may be developed. Methods A single-institution retrospective cohort analysis was performed on patients who underwent pancreatic ductal adenocarcinoma resection between 2011 and 2015. Clinical, operative and pathological data were analyzed. Statistical significance was defined as a p value <0.05. Results A total of 128 patients with pancreatic ductal adenocarcinoma underwent surgical resection in this study period. Recurrence was observed in 82 (64%) patients. Overall median time to recurrence was 12.1 months. The most common site of recurrence within six months after surgery was observed in the liver. Earliest recurrence was most frequently seen in the peritoneal cavity and liver, and longest time to recurrence was found in the lung. On Cox regression analysis, perineural invasion was identified as an independent predictor of shorter disease free survival. Survival also significantly differed by sites of recurrence, with the shortest survival observed in patients with peritoneal recurrence, and the longest survival observed in patients with lung recurrence. Conclusion This retrospective study demonstrates various patterns of recurrence following surgical resection of pancreatic ductal adenocarcinoma. By better understanding of recurrence patterns, disease progression can be better predicted and follow up can be tailored accordingly.
Adenocarcinoma; Pancreas; Recurrence; Survival
CT computed tomography; ERCP endoscopic retrograde cholangiopancreatography; EUS endoscopic ultrasound; FDG-PET fluorodeoxyglucose positron emission tomography; PDAC pancreatic ductal adenocarcinoma; NA neoadjuvant
Pancreatic ductal adenocarcinoma is currently the fourth leading cause of cancer death worldwide [1, 2]. Despite improvements in surgical technique, perioperative care, systemic treatment and follow-up methodology, PDAC continues to have dismal 5-year survival of only 5% with a reported median survival of 6 months [3, 4, 5, 6]. Surgical resection is still considered the only potentially curative treatment modality in selected patients. However, the vast majority of patients with resected PDAC either recur locally or have distant metastases within two years [7, 8, 9, 10].
Over the last two decades, multiple studies have explored clinicopathological variables that may help identify patients who are more likely to have a recurrence of PDAC following surgery [11, 12, 13, 14]. However, the timeline to recurrence and the different patterns of local vs. organ-specific recurrence based on clinicopathological variables has not been well characterized. A recent study by Groot et al. provided some insight but excluded patients who received neoadjuvant (NA) chemotherapy [10]. Unfortunately, this excludes a significant cohort of patients with borderline resectable disease who would normally be recommended NA chemotherapy or chemo-radiotherapy at high-volume centers [15].
This study aims to investigate 1) the timeline and pattern of recurrence for all patients following surgical resection of PDAC and 2) the impact of the site of recurrence on survival.
Study Population
Patients undergoing pancreatic resection for nonmetastatic histopathologically confirmed PDAC at Royal North Shore Hospital and North Shore Private Hospital (Sydney, NSW, Australia) between January 2011 and December 2015 were identified from a prospectively maintained database. All patients were assessed at a multidisciplinary meeting and received routine preoperative workup including: triple phase computed tomography (CT) of the abdomen and pelvis; CT chest; endoscopic ultrasound (EUS) or endoscopic retrograde cholangiopancreatography (ERCP) for cytology; and a staging laparoscopy. Patients with distant lesions equivocal for metastatic spread on CT imaging underwent a 18-fluorodeoxyglucose positron emission tomography (FDG-PET) scan. Distant FDG-avid lesions were subsequently biopsied. In patients with non-metastatic disease, pancreatic resection was performed according to previously described methods [16, 17, 18]. Patients with non-PDAC periampullary lesions (e.g. ampullary adenocarcinoma, distal cholangiocarcinoma, duodenal cancer, neuroendocrine tumors, cystic neoplasms) were excluded from analysis. Patients with incomplete followup data were also excluded. The final cohort of patients who underwent curative-intent pancreatic resection for PDAC was retrospectively analyzed.
Demographic and Clinicopathological Characteristics
Patient demographics and pathological variables were obtained from the departmental database. Details regarding the use of NA and/or adjuvant therapy were recorded. Patients with borderline resectable disease according to the 2016 Australasian Gastrointestinal Trials Group Guidelines [19] were referred for NA therapy administered according to the recommendation of the multidisciplinary team. NA therapy included six months of perioperative chemotherapy (gemcitabine only, gemcitabine and nab-paclitaxel, or FOLFIRINOX) with/ without NA radiotherapy. The following pathological variables were analyzed: tumor size, resection margin status, lymphovascular and perineural invasion, and lymph node status. The largest diameter of resected specimen was taken as the tumor size. The location of the nearest margin was recorded as follows: portal vein bed, periuncinate soft tissue, pancreatic neck, anterior pancreatic capsule and posterior surface. An R1 resection was classified as a resection margin of ≤ 1 mm according to the standardized protocol for PDAC histology reporting [20]. The presence or absence of small vessel invasion, large vessel invasion and perineural invasion was identified. Classification of small and large vessel invasion was based on the ‘Structured Reporting Protocol’ by the Royal College of Pathology of Australasia [21]. Absolute lymph node count was determined from the resected specimen, and the lymph node ratio was calculated as the number of lymph nodes involved by tumor divided by the total number of resected lymph nodes. Overall survival was defined as a period from the date of surgery to the date of death.
Follow-up and PDAC Recurrence
Patients were followed up in an outpatient setting by the multidisciplinary team including surgeons, medical oncologists, and radiation oncologists. Follow-up evaluation included history, physical examination every three to six months for the first two years monitoring serum cancer-associated antigen 19-9 (CA 19-9) levels and CT scans of the chest, abdomen and pelvis. In the absence of recurrent disease after two years, patients were followed up annually. Follow up data collection concluded on January 17 2017. Disease recurrence was determined by evidence of a malignant appearing lesion on CT with histological/cytological confirmation or an 18- FDG-avid lesion on FDG-PET scan. A local recurrence was defined as the development of recurrent malignant disease within the pancreatic bed or regional lymph nodes. Distant recurrence was defined as a metastatic PDAC lesion developing outside of the pancreatic bed or regional lymph nodes, including the liver, lung, peritoneum, bone and abdominal wall. Multiple recurrence was defined as the simultaneous diagnosis of recurrent PDAC occurring in more than one body site, whether local or distant.
Ethics
This study was approved by the Northern Sydney Local Health District and North Shore Private Hospital Ethics Committees (Study numbers HREC/16/HAWKE/105 and NSPHEC 2016-007).
Statistical Analysis
Survival analysis was performed using the Kaplan- Meier method, and differences in survival were compared using the log-rank test. A Cox regression analysis was performed to identify factors independently associated with recurrence. A p-value of <0.05 was considered to be statistically significant.
Demographics
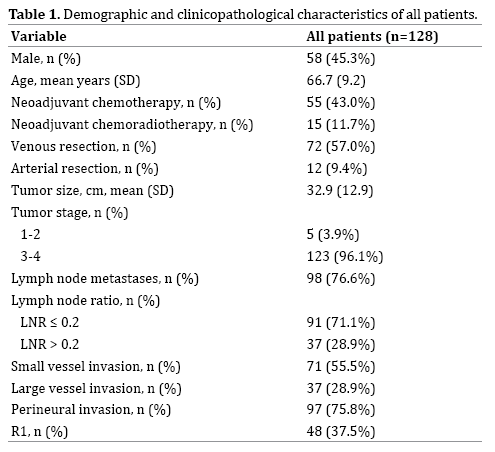
A total of 128 patients with histologically diagnosed PDAC underwent surgical resection in the study period. Seventy (55%) patients were female. The median age at the time of operation was 68 (IQR 60-73). Resection type was pancreaticoduodenectomy in 115 (89.8%), distal pancreatectomy in eight (6.3%) and total pancreatectomy in five patients (3.9%). Seventy patients (54.7%) received NA therapy of which 55 (42.9%) received NA chemotherapy and 15 (11.7%) received NA chemoradiotherapy. Of those who received NA chemotherapy, 37 patients developed disease recurrence. Eight patients who had NA chemoradiotherapy developed disease recurrence. The demographic and clinicopathological characteristics are summarized in Table 1. The median follow-up for all patients was 15.5 months (IQR 9-25.25 months). Disease recurrence was observed in a total of 82 (64%) patients (Table 1).
Factors Associated with Recurrence
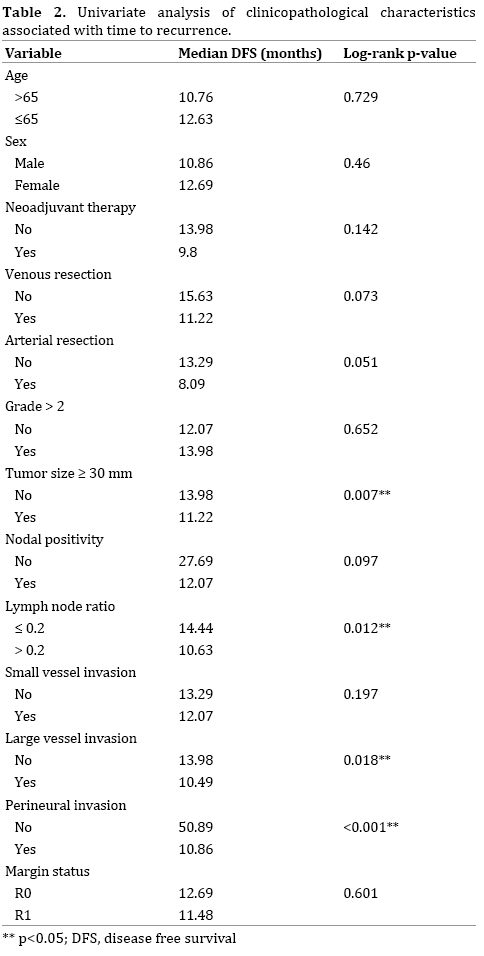
Univariate analysis of clinicopathological characteristics associated with time to recurrence is detailed in Table 2. Factors significantly associated with shorter median diseasefree survival (DFS) on this analysis included: tumor size ≥ 30 mm, lymph node ratio >0.2, large vessel invasion, and perineural invasion. Patients requiring a venous or arterial resection were associated with a non-significant trend towards shorter disease free survival (DFS).
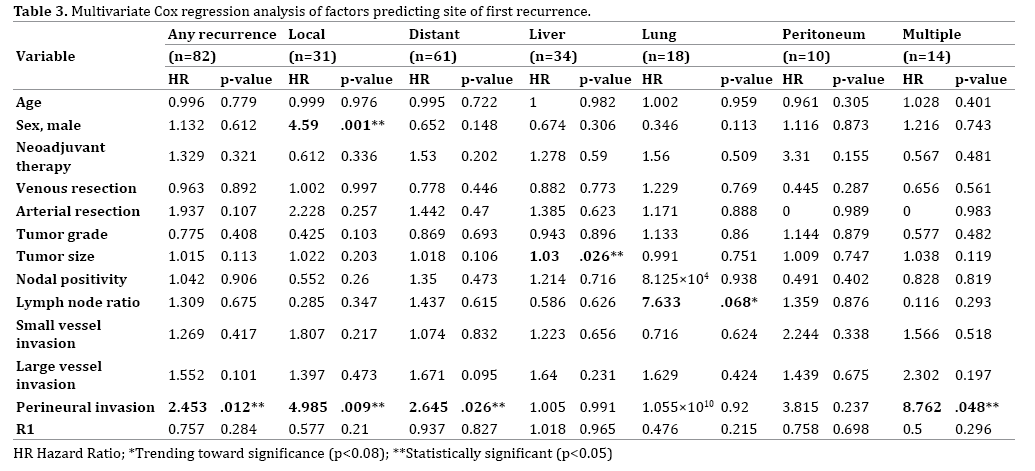
Multivariate Cox regression analysis was performed to identify independent predictors of disease recurrence (Table 3). Perineural invasion was identified as a significant independent predictor of early disease recurrence at any site (HR 2.43, p=0.012).
Site of Recurrence
Clinicopathological characteristics associated with the site of first recurrence of PDAC are shown on Table 3. Thirty-one patients (24.2%) developed a local recurrence, and 61 patients (47.7%) developed a distant metastatic recurrence. Thirty-four patients (26.6%) developed liver metastases, 18 (14.0%) developed lung metastases, and 10 (7.8%) developed peritoneal metastases. Fourteen patients (10.9%) developed recurrence in multiple body sites simultaneously.
Of the patients who developed multiple recurrences, the majority of patients (n=10/14) developed local recurrence with one other site of distant metastasis. When discovered simultaneously with other sites of recurrent disease, liver metastases were found most often in combination with local recurrences (n=6/10) followed by peritoneal lesions (n=3/10).
On Cox regression analysis, factors significantly predictive of the development of local recurrence included male gender (HR 4.590, p=0.001) and the presence of perineural invasion (HR 4.985, p=0.009). Perineural invasion was the only factor independently associated with the development of distant recurrence (HR 2.645, p=0.026). Increasing tumor size was significantly predictive of the development of liver recurrence (HR 1.030, p=0.026). An increase in lymph node ratio was associated with a trend toward an association with the development of lung metastases (HR 7.633, p=0.068). There were no factors predictive of the development of peritoneal recurrence. Perineural invasion was also significantly associated with the development of multiple simultaneous recurrences (HR 8.762, p=0.048).
Disease Recurrence and Survival
The median overall survival (OS) for the entire cohort was 26.2 months (IQR 12.6 – 57.4 months). Median OS was significantly longer in patients without recurrence as compared with those who did experience recurrence of disease (53.5 vs. 20.1months, log-rank p<0.001). Survival curves are presented in Figure 1a.
The median disease free survival was 12.1 months (IQR 7.3 - 36.5 months). There was a significant difference in median time to recurrence depending on the site of first recurrence (Figure 1b). Median time to recurrence according to site of recurrence were: peritoneal, 2.7 months (IQR 1.6-3.5 months); multiple sites, 6.7 months (IQR 5.1- 14.4 months); liver, 7 months (IQR 4.1-11.8 months); local, 10.4 months (IQR 7.8-12.7 months); and lung, 11.5 months (IQR 7.9-30.1 months, log-rank p=0.001) (Figure 1b). The combined timeline to recurrence is represented in Figure 2.
Median survival with recurrent disease (date of recurrence to date of death/censor) was 7.8 months (IQR 3.7-18.5 months). There was a significant difference in median survival with recurrent disease depending on the site of recurrence. Median survival periods with recurrent disease according to site of recurrence were: peritoneal, 2.8 months (IQR 2.1-4.1 months); multiple sites, 3.9 months (IQR 2.8-5.2 months); liver, 7.6 months (IQR 3.8-18.5 months); local, 11.4 months (IQR 6.3-30.9 months); lung, 12.9 months (IQR 6.1-30.4 months, log-rank p=0.007).
The current study reviewed the pattern and site of recurrence in resected PDAC patients including those who received NA therapy and identified clinicopathological predictors of recurrence. On univariate analysis, larger tumor size, large vessel invasion, higher lymph node ratio and perineural invasion were significantly associated with shorter DFS. On multivariate Cox regression analysis, perineural invasion was independently associated with shorter DFS. This is in keeping with Ozaki et al. [22] and Lewis et al. [23]. Several clinicopathological factors were identified as independent risk factors for the development of recurrence in particular sites of the body. Notably, male gender was significantly associated with the development of local recurrence, and perineural invasion was significantly associated with the development of local, distant, and multiple recurrences. This study also demonstrated significantly different survival durations in the presence of recurrence depending upon the location of recurrence. Patients with peritoneal recurrence had the shortest median survival in the presence of recurrence, and those with local or lung recurrence survived the longest duration.
The present study largely confirms the findings of previously published studies examining similar variables and outcomes. A large tumor size and a greater lymph node burden increase the risk of disease recurrence and poorer survival [10, 24, 25, 26, 27]. The distribution and timing of sites of recurrence in the present study are very similar to those reported by Groot et al.
Contrary to some published reports, the present study does not demonstrate a significant relationship between R1 resection and the risk of local recurrence [24, 25, 26]. Van de Broek et al. [11] and Groot et al. [10] showed a significant reduction in survival in patients with R1 resection and increased local recurrence rates. However, our findings are in agreement with Nitta et al. who reported that there was no increase in the local recurrence rates in R1 resections [28]. The figures in the present study may be attributed to high rates of NA as well as adjuvant chemotherapy which has been shown to decrease local recurrence rates significantly [29, 30].
There was a distinct survival pattern for individual sites of recurrence in our series. The current study has demonstrated significantly longer disease-free survival and overall better prognosis in patients who developed solitary lung recurrences as the first site of recurrence. This is in contrast to patients with liver, peritoneal and multiple sites of recurrence who had shorter disease-free survival and shorter overall survival (Figure 1b). Recent studies [3, 9, 31] have also observed a similar pattern of better prognosis among patients who developed solitary lung recurrences after PDAC resection [10]. Whether the pattern of recurrence in PDAC reflects a particular pattern of gene expression remains to be elucidated. Campbell et al. have demonstrated particular PDAC genotypes can drive metastases to particular organs, which may explain organ-specific metastases [32]. Identification of genomic, proteomic, and/or metabolomic signatures predicting individual patterns of recurrence may significantly influence early recognition and tailored therapy.
The significant differences demonstrated in the timing of recurrence depending on the site of recurrence may have implications on the way that patients are surveyed post-operatively. The data from the present study suggest that the highest risk for peritoneal recurrence is within the first three months, whereas local and lung recurrence occur much later. Practically, this implies that the clinician should remain vigilant for the development of peritoneal recurrence particularly in the early follow-up period. Rising serum Ca19-9 levels in the absence of visible recurrence on CT imaging should prompt an 18-FDG-PET scan to look for peritoneal disease.
The identification of particular factors associated with patterns of recurrence also carries clinical implications. Patients with perineural invasion in particular may warrant a more intensive follow-up schedule. From the data in the present study, it is unclear why males should be more likely to develop local recurrence. Further work is required to determine whether this relates to unknown factors that were not included in the multivariate model e.g. compliance with systemic therapy or genetic/epigenetic phenomena.
This current study included patients who underwent NA therapy prior to resection. It is understood that NA therapy controls local disease and is thereby able to downstage patients with locally advanced PDAC to resectable criteria [27, 33]. This ultimately influences prognosis by providing an opportunity for surgical resection which is the only significant treatment known to have a chance of cure. In the literature, it has been suggested that NA therapy substantially lowers the rate of local recurrence following resection [30, 34, 35].
Currently, most patients with disease recurrence are managed with cytotoxic chemotherapy regardless of the site of recurrence. Whilst this may be appropriate for patients with evidence of widespread disease, locoregional or organ-specific therapies may be suitable for patients with single site disease. Examples of such therapies include radiofrequency ablation, transarterial chemoembolization (TACE) and SIR-Spheres for liveronly disease, and radiotherapy for local recurrence [36, 37]. Surgical resection of isolated metastases has also been recently reported with inconsistent reports of survival benefit [38, 39]. Given the paucity of highquality data to guide such management decisions, further research in the form of randomized trials are required to clarify the efficacy of these locoregional management strategies.
There were several limitations to our study. While the cohort size was small, the follow-up was very close thereby capturing the majority of patients as opposed to other studies where only a small percentage of the cohort could be included for analysis [10]. A longer follow-up period may have captured future recurrences for some patients which may take longer to develop such as lung recurrences. The current study did not include quality of life data, which should be the focus of future pancreatic cancer research. The inclusion of how the disease recurrence and the subsequent treatment affects the patients' and their family's quality of life is important to enable more comprehensive end of life care.
The rate of PDAC recurrence following curative resection remains high. There are distinctive patterns of recurrence by the site which inevitably also affects survival. Further research is now needed to investigate possible tumor cell and or stroma specific biomarkers which may help predict these patterns of disease recurrence. Such research will be critical for personalizing cancer therapy for the PDAC patient.
We declare that we have no conflict of interests.