Original Article - (2017) Volume 18, Issue 1
Yusuke Watanabe1, Kazuyoshi Nishihara1, Yusuke Niina2, Yuzan Kudo1, Kanako Kurata1, Takafumi Okayama1, Atsushi Fujii1, Shinichi Wakamatsu3, Yuji Abe1, Toru Nakano1
Departments of 1Surgery, 2Gastroenterology, and 3Oncology, Kitakyushu Municipal Medical Center, 2-1- 1, Bashaku, Kokurakita-ku, Kitakyushu 802-0077, Japan
Received October 10th, 2016 - Accepted November 10th, 2016
Purpose To investigate recent recurrence patterns after curative resection for pancreatic ductal adenocarcinoma and the association between the recurrence pattern and prognosis. Methods We retrospectively reviewed the medical records of 129 patients who underwent curative resection for pancreatic ductal adenocarcinoma. Results Of the 129 patients, 90 developed recurrence. Recurrence occurred in the liver in 45 patients (50%), lung in 11 (12%), peritoneum in 34 (38%), and local tissue in 26 (29%). The number of patients with lung recurrence was significantly higher in the second half of the study period (10/57, 18%) than in the first half (1/33, 3%; P=0.04). Adjuvant chemotherapy was more frequently performed in patients with lung recurrence (9/11, 82%) than in those with recurrence at other sites (40/79, 51%; P=0.04). Patients with lung recurrence only (n=8) had a significantly better prognosis than those with other patterns of recurrence (n=82) in terms of recurrence-free survival (P=0.02), time from recurrence to death (P=0.02), and overall survival (P<0.01). Multivariate analysis showed that lung recurrence was an independent favorable factor for the time from recurrence to death (HR: 0.09; 95% CI: 0.02–0.35) and overall survival (HR: 0.12; 95% CI: 0.03–0.40). Conclusions The incidence of lung recurrence after pancreatectomy for pancreatic ductal adenocarcinoma has recently increased, which may be due to the recent establishment of adjuvant chemotherapy. Patients with lung recurrence have a better prognosis than those with recurrence at other sites.
Adenocarcinoma; Pancreatectomy, Prognosis
OS overall survival; PD pancreatoduodenectomy; PDAC pancreatic ductal adenocarcinoma; RFS recurrence-free survival; RTD time from recurrence to death
The prognosis for pancreatic ductal adenocarcinoma (PDAC) remains dismal despite recent advances in multimodal treatment. The only potentially curative treatment for PDAC is surgical resection; however, the prognosis even after resection remains unsatisfactory, with a reported 5-year survival rate after resection of 18% to 27% [1, 2, 3, 4]. Approximately 80% of surgically resected PDACs recur within 5 years after resection, and >60% of patients develop recurrence within 2 years [3, 4, 5, 6].
Adjuvant chemotherapy after resection for PDAC has recently become a general therapeutic strategy to prevent recurrence and improve the prognosis [6, 7]. Prior to the use of adjuvant chemotherapy, recurrence sites after resection for PDAC included the liver, peritoneum, and local tissue, accounting for 97% of total recurrences [5, 8]. Following the generalized use of adjuvant chemotherapy, recent recurrence patterns after resection for PDAC may have changed; however, these changes are not well documented. Some investigators have reported that patients with lung metastasis after resection for PDAC have a better prognosis than those with recurrence in other sites [5, 9]; however, these reports included many patients who underwent resection with residual microscopic or macroscopic disease (R1 or R2 resection), although R1 or R2 resection is a well-documented factor associated with poor outcomes and may cause early local or peritoneal recurrence [10, 11, 12].
A good understanding of recent recurrence patterns after curative resection for PDAC without residual microscopic or macroscopic disease (R0 resection) as well as the association between the recurrence pattern and the prognosis may provide keys to improving the dismal prognosis of PDAC. The aim of this study was to elucidate the recent recurrence patterns after curative resection for PDAC and the association between the recurrence pattern and prognosis.
This retrospective study was approved by the Ethics Committee of Kitakyushu Municipal Medical Center (approval number 201506009). We retrospectively reviewed the medical records of 166 consecutive patients who underwent pancreatectomy for PDAC at our institution from 1996 to 2015. The residual tumor status was defined according to the Union for International Cancer Control (UICC) classification as follows: R0 resection, no residual tumor; R1 resection, microscopic residual tumor as indicated by the presence of tumor cells at the surface of the resection margin; and R2 resection, macroscopic residual tumor [13]. In this study, curative resection for PDAC was defined as R0 pancreatectomy, including pancreatoduodenectomy (PD), distal pancreatectomy, or total pancreatectomy, with regional lymph node dissection. We excluded patients who underwent R1 or R2 resection and those who underwent non-typical pancreatectomy.
We evaluated the patients’ demographic, clinical, intraoperative, postoperative, pathological, and survival data. The patients’ demographic and clinical data included age, gender, and preoperative serum CEA and CA19-9 concentrations. Aggressive preoperative therapy, such as neoadjuvant chemotherapy or radiotherapy, was not performed during the study period. Intraoperative data included the type of surgery, portal vein resection and reconstruction, and transfusion. Postoperative data included mortality and adjuvant chemotherapy, with postoperative mortality defined as death within 30 days or in-hospital death. We also excluded patients who died postoperatively.
The indication for adjuvant chemotherapy and the type, dosage, and duration of drugs prescribed were decided by the attending physicians before 2006. Our institution introduced adjuvant chemotherapy using gemcitabine as a standard therapeutic strategy from 2007, and tegafur/ gimeracil/oteracil potassium (S1) was also used from 2013. In principle, adjuvant chemotherapy was indicated for all patients who underwent pancreatectomy for PDAC from 2007 onward; however, the administration and dosage of adjuvant chemotherapy were ultimately decided by the attending physicians, according to the patient’s condition or wish. Patients treated with gemcitabine received intravenous gemcitabine at a dose of 1000 mg/ m2 on days 1, 8, and 15 (once a week for 3 weeks) followed by a 1-week rest period (one cycle). This administration of gemcitabine was repeated every 4 weeks for up to six cycles. Patients treated with S1 received oral S1 at a dose of 40 mg for a body surface area of <1.25 m2, 50 mg for a body surface area of 1.25 to 1.49 m2, and 60 mg for a body surface area of ≥1.50 m2 twice per day for 28 consecutive days followed by a 14-day rest period (one cycle). This administration of S1 was repeated every 6 weeks for up to four cycles.
During the study period, follow-up examinations were performed using contrast-enhanced whole-body CT and tumor marker testing, including measurement of the CEA and CA19-9 concentrations, every 3 months until 2 years after pancreatectomy. Follow-up examinations were then performed every 6 months until 10 years after pancreatectomy. When a patient complained of symptoms suggestive of recurrence, contrast-enhanced whole-body CT and tumor marker testing were performed accordingly. Therefore, when tumor recurrence was detected, contrastenhanced whole-body CT was performed to assess whether single-organ recurrence or synchronous multiorgan recurrence was present. MRI or PET/CT was used as a complementary technique to diagnose recurrence. Survival data included the presence of recurrence, related data, the first site of recurrence, and whether the patient was alive or dead. The last month of follow-up was January 2016.
Pathological data included tumor size, tumor differentiation (G-status), vascular invasion, lymphatic invasion, and perineural invasion. The G-status was defined as follows: G1, well-differentiated tumor; G2, moderately differentiated tumor; and G3, poorly-differentiated tumor [13]. The pathological tumor stage was determined according to the UICC TNM classification [13].
Statistical analysis was performed using JMP statistical software (version 9.0.2; SAS Institute, Cary, NC). Continuous data are presented as median and range. The Mann–Whitney U-test was used to assess continuous data. Fisher’s exact test or the chi-squared test was used to evaluate differences in categorical data. Recurrence-free survival (RFS) was defined as the time from the date of pancreatectomy to recurrence, and patients alive without recurrence at the last follow-up were censored. The time from recurrence to death (RTD) was defined as the time from the date of recurrence to death of any cause, and patients alive at the last follow-up were censored. Overall survival (OS) was defined as the time from the date of pancreatectomy to death of any cause, and patients alive at the last follow-up were censored. Survival was calculated using the Kaplan–Meier method and compared between the groups by the log-rank test. A multivariate survival analysis was performed using a Cox proportional hazard model. A P value of <0.05 was considered significant.
Of 166 patients who underwent pancreatectomy for PDAC, we excluded 34 patients who underwent R1 or R2 resection and 1 patient who underwent non-typical middle pancreatectomy. Two patients who died postoperatively were also excluded. Therefore, 129 patients were enrolled in this study. Of these 129 patients, 90 developed recurrence. The characteristics of all patients and those with recurrence are shown in Table 1. Among 90 patients with recurrence, the first site of recurrence was the liver in 45 (50%) patients (liver only in 29), lung in 11 (12%) (lung only in 8), peritoneum in 34 (38%) (peritoneum only in 12), local tissues in 26 (29%) (local only in 12), bone in 2 (2%) (bone only in 1), and kidney only in 1 (1%). When recurrence was diagnosed, 63 (70%) patients had singleorgan recurrence, whereas 27 (30%) had synchronous multi-organ recurrence. The characteristics of patients with recurrence according to the first site of recurrence are shown in Table 2. Adjuvant chemotherapy was more frequently performed in patients with lung recurrence (9/11, 82%) than in those with recurrence at other sites (40/79, 51%; P=0.04). Perineural invasion was more frequent in patients with peritoneal recurrence (30/34, 88%) than in those with recurrence at other sites (34/56, 61%; P<0.01). There were no significant differences in other baseline characteristics.
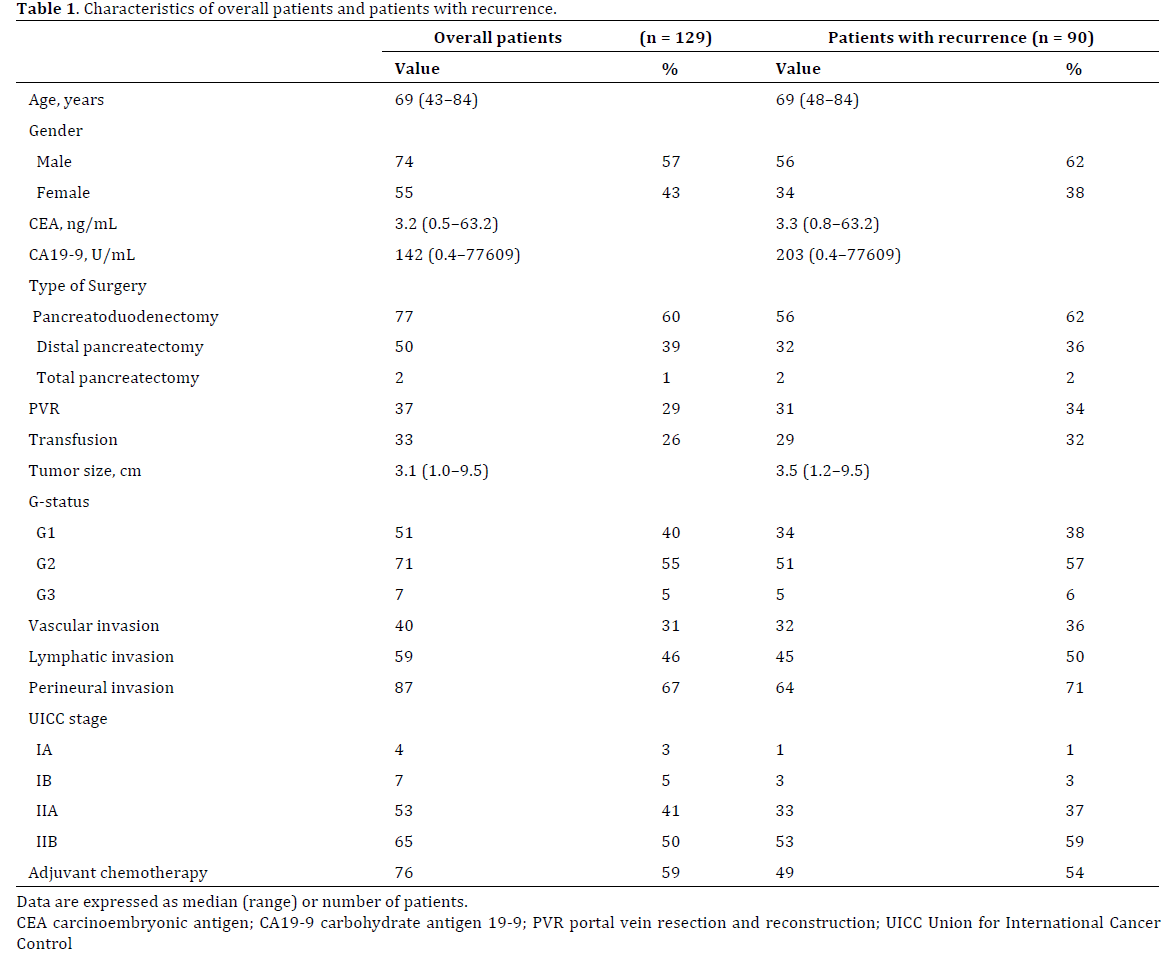
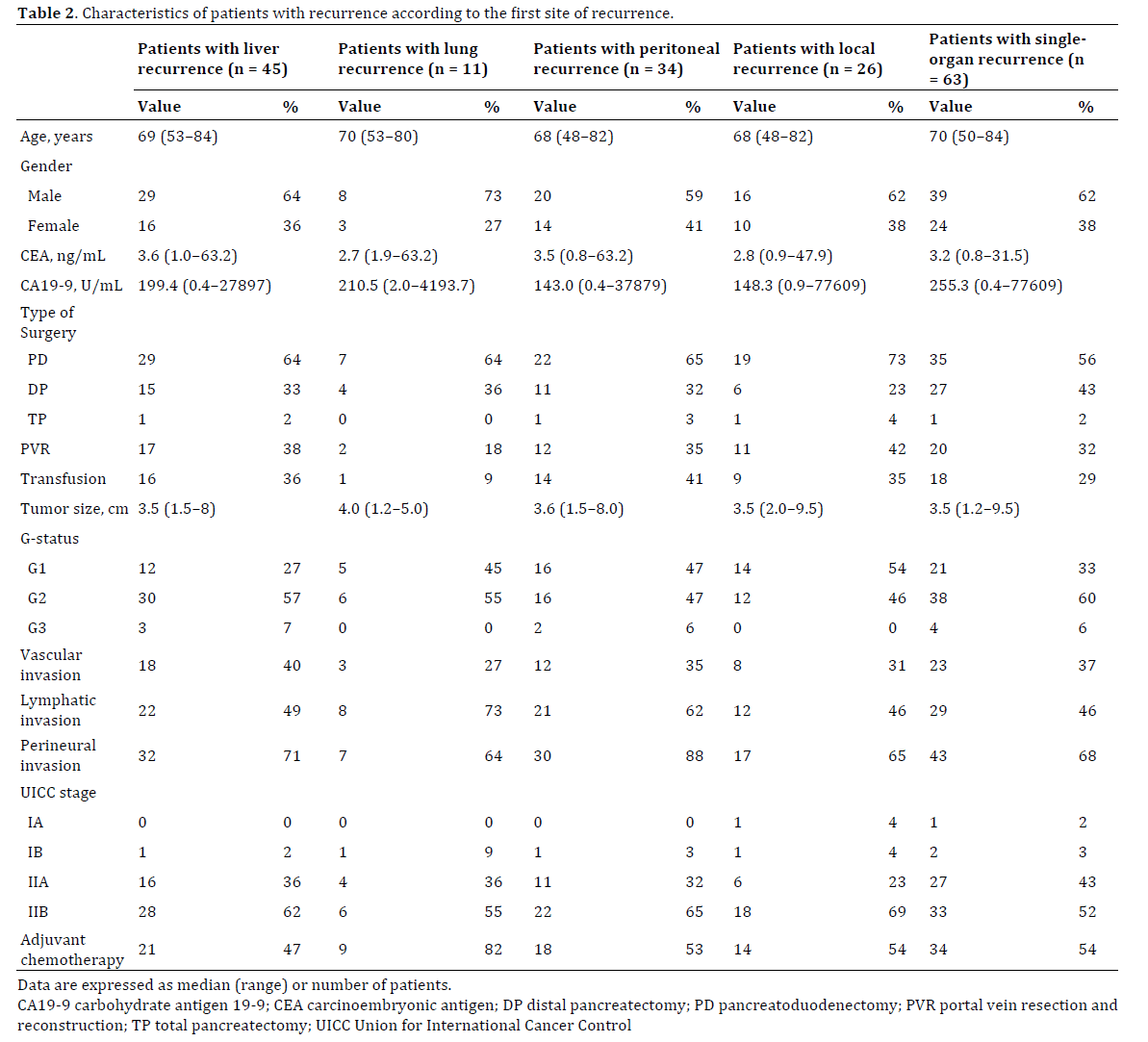
When the study population was divided by the date of pancreatectomy into the first half of the study period (1996–2006; n=33) and the second half (2007–2015; n=57), the number of patients with lung recurrence was significantly higher in the second half (10/57, 18%) than in the first half (1/33, 3%; P=0.04), whereas the number of patients with peritoneal recurrence was significantly lower in the second half (17/57, 30%) than in the first half (17/33, 52%; P=0.04). There was no significant difference in the prevalence of liver recurrence between the first and second halves of the study period (first half: 17/33 (52%) vs. second half: 38/57 (49%); P=1.00), local recurrence (first half: 10/33 (30%) vs. second half: 16/57 (28%); P=0.81), or multi-organ recurrence (first half: 12/23 (36%) vs. second half: 15/57 (26%); P=0.35, respectively).
All patients were followed-up completely during a median period of 19 months (range, 3–226 months). The median RFS duration was 13 months, and the 3- and 5-year RFS rates were 30% and 24%, respectively. The median RTD was 7 months, and the 3-year RTD rate was 6%. The median OS duration was 21 months, and the 3- and 5-year OS rates were 37% and 28%, respectively. Patients with lung recurrence only (n=8) had a significantly better prognosis than those with other patterns of recurrence (n=82) in terms of RFS (P=0.02), RTD (P=0.02), and OS (P<0.01) (Figure 1). Detailed survival analyses among patients with liver recurrence only (n=29), lung recurrence only (n=8), peritoneal recurrence only (n=12), local recurrence only (n=12), and multi-organ recurrence (n=27) at the time of diagnosis of recurrence are shown in Figure 2. Survival analyses according to the first site of recurrence are shown in Table 3. Patients with lung recurrence (n=11) had a significantly better prognosis in terms of RTD and OS. In contrast, patients with liver recurrence had a significantly worse prognosis in terms of RFS and OS, and those with peritoneal recurrence had a significantly worse prognosis in terms of RTD. Patients with multi-organ recurrence had a significantly worse prognosis for RFS, RTD, and OS. An additional survival analysis of 57 patients who underwent curative resection during the second half of the study period and who developed recurrence (n=57) is shown in Figure 3. Patients with lung recurrence only (n=7) had a significantly better prognosis than those with other patterns of recurrence (n=50) in terms of RFS (P=0.01), RTD (P=0.04), and OS (P<0.01).
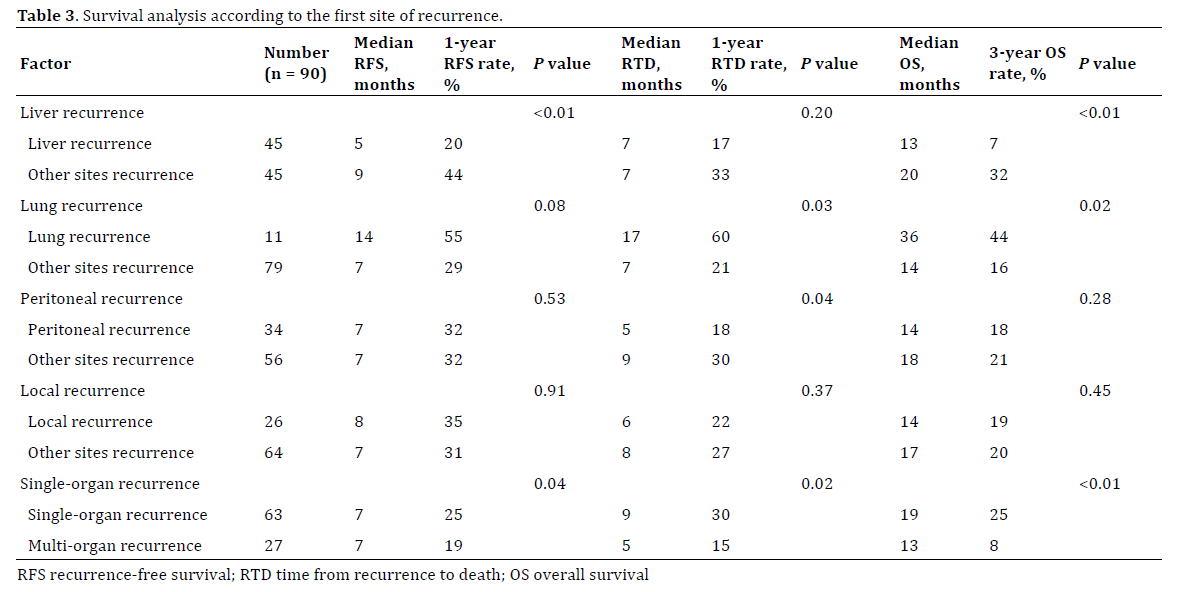
Figure 2. Survival curves according to the first site of recurrence. (a). Recurrence-free survival. Using patients with lung recurrence only as a reference for comparison, the hazard ratios are 4.2 (95% CI: 1.9–10.8, P<0.01) for patients with liver recurrence only, 1.1 (95% CI: 0.5–3.0, P=0.78) for patients with peritoneal recurrence only, 2.1 (95% CI: 0.8–5.7, P=0.11) for patients with local recurrence only, and 4.0 (95% CI: 1.7–10.4, P <0.01) for patients with multi-organ recurrence. (b). Time from recurrence to death. Using patients with lung recurrence only as a reference for comparison, the hazard ratios are 2.2 (95% CI: 0.9–6.7, P=0.08) for patients with liver recurrence only, 3.3 (95% CI: 1.2–10.6, P=0.02) for patients with peritoneal recurrence only, 2.4 (95% CI: 0.8–7.8, P=0.10) for patients with local recurrence only, and 3.7 (95% CI: 1.5–10.9, P<0.01) for patients with multi-organ recurrence. (c). Overall survival. Using patients with lung recurrence only as a reference for comparison, the hazard ratios are 3.6 (95% CI: 1.5–10.9, P<0.01) for patients with liver recurrence only, 2.2 (95% CI: 0.8–6.9, P=0.14) for patients with peritoneal recurrence only, 2.7 (95% CI: 1.0–8.9, P=0.06) for patients with local recurrence only, and 5.2 (95% CI: 2.1–15.7, P<0.01) for patients with multi-organ recurrence.
Figure 3. Survival curves of patients who underwent curative resection during the second half of the study period. Patients with lung recurrence only (n=7) had a significantly better prognosis than those with other patterns of recurrence. (a). Recurrence-free survival (P=0.01). (b). Time from recurrence to death (P=0.04). (c). Overall survival (P<0.01).
Multivariate analyses of favorable prognostic factors for survival are shown in Table 4. Single-organ recurrence was an independent favorable factor for RFS, RTD, and OS. Lung recurrence was an independent favorable factor for RTD and OS.
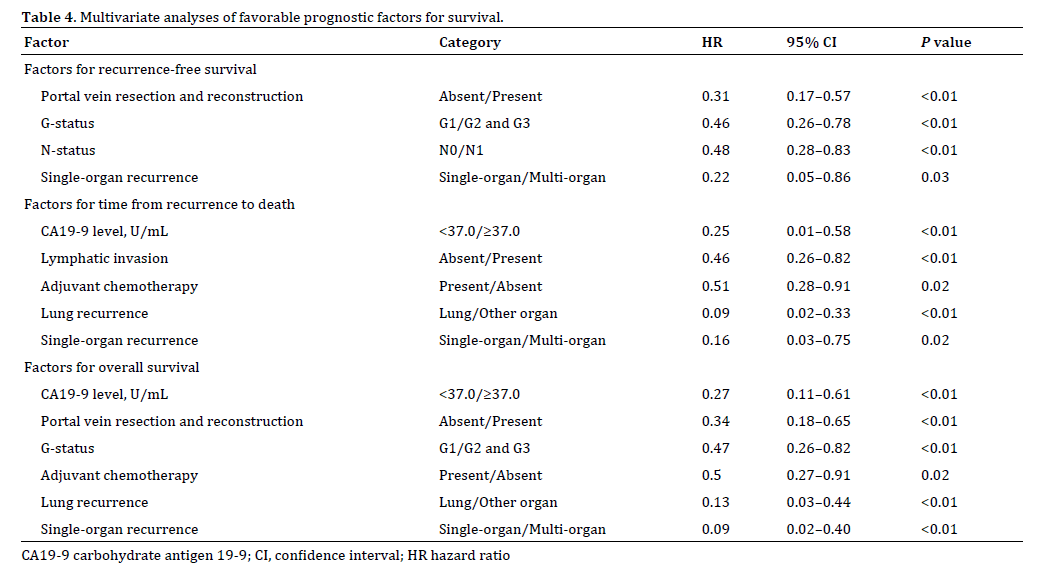
This study revealed the following findings: (1) Recent recurrence patterns after curative resection for PDAC have changed. The incidence of lung recurrence has increased, whereas the incidence of peritoneal recurrence has decreased. (2) Patients with lung recurrence after curative resection for PDAC had a significantly better prognosis, and lung recurrence was an independent favorable factor for RTD and OS. (3) Patients with multi-organ recurrence had a significantly worse prognosis, and single-organ recurrence was an independent favorable factor for RFS, RTD, and OS.
Our results show that the prevalence of lung recurrence after curative resection for PDAC has increased in recent years. The prevalence of lung recurrence after 2007 was 18% and much higher than that reported historically [5, 8]. Adjuvant chemotherapy was more frequently performed in patients with lung recurrence than in those with recurrence at other sites. In Japan, adjuvant chemotherapy using gemcitabine was generally introduced in 2007, with the use of S1 recommended from 2013 [14]. The number of patients with lung recurrence increased after 2007 in this study, suggesting that the recent generalized use of adjuvant chemotherapy may have resulted in a change in the recurrence patterns after curative resection for PDAC.
This study revealed that patients with lung recurrence after curative resection for PDAC had a significantly better prognosis and that lung recurrence was an independent favorable factor for RTD and OS on multivariate analysis. This retrospective study was performed over a long period, and historical bias was a concern. Therefore, we performed an additional survival analysis of patients who underwent curative resection during the second half of the study period and who developed recurrence because our institution introduced adjuvant chemotherapy as a standard therapeutic strategy from 2007. Of patients who underwent resection during the second half of the study period and who received adjuvant chemotherapy as a standard therapeutic strategy, those with lung metastasis also had a significantly better prognosis. The type of PDAC that recurs in the lung may have distinct biological features that provide keys to improving the dismal prognosis of PDAC. Our results also showed that single-organ recurrence was an independent favorable factor for RFS, RTD, and OS. In selected patients with colorectal carcinoma, resection for isolated liver or lung metastasis is recommended to improve the prognosis [15, 16], and some investigators have suggested that pulmonary resection for isolated metastasis of PDAC may improve survival [17, 18]. Pulmonary resection for isolated metastasis of PDAC to the lung may be an optional treatment strategy for PDAC.
A few studies have investigated the association between the recurrence pattern after resection for PDAC and prognosis [5, 9]. Wangjam et al. [5] and Downs- Canner et al. [9] reported that PDAC with lung recurrence had a significantly better prognosis than recurrence at other sites; however, these studies included patients who underwent R1 or R2 resection. Several recent studies of the prognosis after pancreatectomy for PDAC included macroscopically curative resection (R0+R1) [19, 20, 21]; however, we recently reported that patients who underwent R1 resection as well as those who underwent R2 resection had a significantly worse prognosis than those who underwent R0 resection [22]. To establish the pure influence of the recurrence pattern on survival, our study included patients who underwent curative R0 resection only; herein lies the novelty of our work.
The limitations of this study are as follows: (1) This was a retrospective study with a small number of patients conducted at a single institution. Moreover, our institution is not a high-volume center for treatment of PDAC. The number of patients was too small to reach a definitive conclusion, and further multi-institutional prospective studies with larger populations are required to assess the true association between recurrence patterns and prognosis. (2) The study population was heterogeneous. This study involved patients who underwent PD, distal pancreatectomy, and total pancreatectomy. Because of the small study population, we included PDAC in different parts of the pancreas. Further studies based on PDAC in each part of the pancreas and surgical procedures of various types are required. (3) We investigated the association between the first site of recurrence and prognosis; however, we did not investigate the association between the number of recurrent lesions at the first recurrence sites and the prognosis because this was a retrospective medical records review. Further prospective studies investigating the relationship between the number of lesions at the first recurrence sites and the prognosis are required. (4) Our follow-up strategy remained consistent; however, the radiological technology and surgical procedures continued to improve during the study period. Because this retrospective study was performed over a long period and historical bias was present, these factors might have affected the changes in the recurrence patterns after pancreatectomy for PDAC. (5) In some previous studies, 75% of patients had indeterminate lung nodules on their surveillance CT prior to the diagnosis of lung recurrence, and differentiation between lung recurrence and inflammatory lesions is difficult [5, 23]. Therefore, the diagnosis of lung recurrence may be delayed. However, we did not evaluate this issue in the present study. Based on these previous reports, the RFS and RTD analyses in our patients with lung recurrence may have been inaccurate, and the prognosis should thus be assessed using only OS.
In conclusion, the incidence of lung recurrence after curative resection for PDAC has increased in recent years, and the cause of the increase may be the recent generalized use of adjuvant chemotherapy. Patients with lung recurrence have a better prognosis than those with recurrence at other sites. Investigation of the biological features of PDAC recurring in the lung may provide keys to improving the dismal prognosis of PDAC.
The authors declare that there is no conflict of interest.