Research Article - (2015) Volume 23, Issue 4
Kelvin Kim BSc
Ran Schwarzkopf MD MSc*
Department of Orthopaedic Surgery, Joint Replacement Service, University of California Irvine, Orange, Ca, USA
Background: Compliance with preoperative guidelines such as medications and body washes are actions a patient can participate in so as to improve outcomes and decrease the risk of adverse events after total joint arthroplasty. The aim of this study was to assess our patients’ compliance with preoperative instructions and guidelines. Proper preoperative compliance might lead to better outcomes in patient safety, care, and overall clinical outcomes of total joint arthroplasty.
Methods: In a prospective observational study, we analyzed patient compliance to a protocolized preoperative regimen that included preoperative warfarin, celecoxib, mupirocin, chlorhexidine body washes, surgical site shaving, and surgical site marking. Consecutive patients undergoing total joint arthroplasty were included. Patients filled out a questionnaire the day of surgery indicating their compliance with the preoperative guidelines. Statistical analysis was completed to calculate confidence intervals of overall compliance and any difference in compliance based on age or gender.
Results: Compliance rates overall were: warfarin 87%, celecoxib 87%, mupirocin 84%, chlorhexidine 98%, shaving 91% and site marking 99%. Based on age sub-group analysis, there was no significant difference in compliance based on age or gender, and compliance did not fall below 82% in any category.
Discussion: The currently used protocol of patient preoperative preparation achieves rates of compliance equivalent if not better to other studies. The lowest overall percentage of compliance was 84% in the mupirocin category. Therefore, the current regimen, while not perfect, is not inferior to other methods of improving patient compliance, and leaves a blueprint for other total joint arthroplasty surgeons to build off.
patient education; preoperative instruction; preoperative medication regimen; patient compliance
Total joint arthroplasty requires a complex orchestration of many medical providers in order to have a successful surgical outcome. Coordination of preoperative, perioperative, and postoperative care is essential for ideal patient outcome and care. However, medical providers are not the only individuals with an effect on surgical outcomes. Patients also play a vital role in their own surgical care and preparedness. Preoperative compliance with a regimen of medications and actions may lead to better patient participation in care as well as clinical outcomes.
The prevailing definition of compliance/adherence is “the extent to which a person’s behavior (in terms of taking medication, following diets, or executing lifestyle changes) coincides with medical or health advice.”[1] Different studies have looked at patient compliance in many different scenarios. The range of patient compliance has varied significantly from 37.5% to 84%.[2-7] Some studies have included a form of education through compliance pledges or different educational classes.[3,5] No previous study has been reported in the literature in regards to patient preoperative regimen compliance in total joint arthroplasty.
Compliance with preoperative guidelines such as medications and body washes are actions a patient can participate in so as to improve outcomes and decrease the risk of adverse events after total joint arthroplasty. Patient education and participation is a central foundation in patient centered care pathways. The aim of this study was to assess our patients’ compliance with preoperative instructions and guidelines. Proper preoperative compliance might lead to better outcomes in patient safety, care, and overall clinical outcomes of total joint arthroplasty.
Design and Patients
Our study was a prospective cohort study of a consecutive group of patients undergoing total hip and total knee arthroplasty at a university-based academic medical center. The study was approved by the institutional review board (IRB).
Inclusion criteria for the study included patients undergoing revision or primary total hip or knee arthroplasty, partaking in the total joint replacement surgical pathway prior to surgery (underwent a structured preoperative visit where instructions were provided), and who had attended the preoperative clinic visit. Exclusion criteria were patients who were not part of the joint replacement surgical pathway such as emergency room admissions, inpatient consults, oncology patients, and those who did not attend the preoperative clinic visit. Exclusion criteria were patients who were not part of the joint replacement surgical pathway such as emergency room admissions, inpatient consults and oncology patients and did not attend the preoperative clinic visit. During patients’ preoperative clinic visit, they were instructed on their preoperative treatment regimen. This included warfarin to be taken the night before surgery, celecoxib to be taken for 2 consecutive nights before surgery, nasal mupirocin application twice a day for five days before surgery if needed for preoperative decolonization of methicillin-resistant Staphylococcus aureus or methicillinsensitive Staphylococcus aureus in the nares), chlorhexidine body wash for three consecutive days before surgery, surgical site shaving, and surgical site marking. Patients were instructed on how to administer and apply the medications prior to surgery, not to shave their surgical site, and not to mark their surgical site before arrival the day of surgery. On the day of surgery, patients were evaluated in regards to compliance with each aforementioned topic with simple “yes” or “no” check boxes as well as amount of days completed for some of the treatments (Appendix I). A registered nurse evaluated patients’ compliance during their preoperative preparation at the operative holding area. The information was recorded in the patients’ charts.
Statistical Analysis
Simple percentages and averages were calculated using the data collected in order to evaluate overall compliance. A logistic regression model was used to calculate odds ratios of likelihood to be compliant based on age groups of 19-50, 51- 75, and 76-94 years. Fisher exact test was used to calculate statistical significance of compliance for gender. In order to meet criteria for compliance each medication was treated slightly differently. For example, celecoxib is contraindicated in patients with a history of gastric or kidney problems, and were instructed not to take them prior to surgery; therefore, patients with gastric or kidney conditions should not be required to take celecoxib in order to be compliant, and as such these patients were not included in the calculation for compliance for these medications. Compliance criteria for warfarin were met if the patient had taken warfarin one day prior to surgery. Compliance criteria for celecoxib were met if the patient had taken celecoxib prior to surgery, or if they abstained from taking celecoxib if they had contraindications. The number of days was then recorded for how many days the patient took celecoxib. The compliance criteria for mupirocin were met if MRSA/MSSA colonized patients applied mupirocin to their nares prior to surgery. The number of days the patient applied the mupirocin was then recorded. The compliance criteria for chlorhexidine were met if patient washed with chlorhexidine prior to surgery. The absolute number of days was recorded of how many days the patient washed with chlorhexidine. The compliance criteria for not shaving the surgical site were met simply if the patient did not shave the surgical site within 3 days of surgery. The compliance criteria for site marking were met if the patient simply did not mark the site prior to surgery with any marking pen/marker.
Statistical analysis was completed with the use of JCP statistical software platform (statpages.org).
Out of 319 consecutive patients that met the inclusion criteria the average age of the patient was 64.0 years old (range of 19- 94 years). 128 males were enrolled compared to 191 females. Among the 319 patients that were included in the cohort, 279 patients took their warfarin prior to surgery which resulted in an 87.46% overall warfarin compliance percentage. 284 patients received celecoxib based on their gastric and kidney- free medical histories, 247 of which were compliant. This resulted in an 86.97% overall celecoxib compliance percentage. 65 of the 77 MRSA/MSSA colonized patients who received mupirocin were compliant, resulting in an 84.41% overall compliance. 314 of 319 used their chlorhexidine scrub, resulting in 98.43% overall chlorhexidine compliance percentage. 289 patients did not shave their surgical site, resulting in 90.60% overall shaving compliance percentage. 315 patients did not mark their surgical site, resulting in 98.75% overall site marking compliance percentage (Figure 1).
Overall, the average number of days among patients taking celecoxib was 1.89 days out of the instructed 2 days with a standard deviation of 0.66 days. Overall, the average number of days among patients using mupirocin was 4.10 days out of the instructed 5 days with a standard deviation of 2.49 days. Overall, the average number of days among patients using chlorhexidine was 2.76 days out of the instructed 3 days with a standard deviation of 0.80 days.
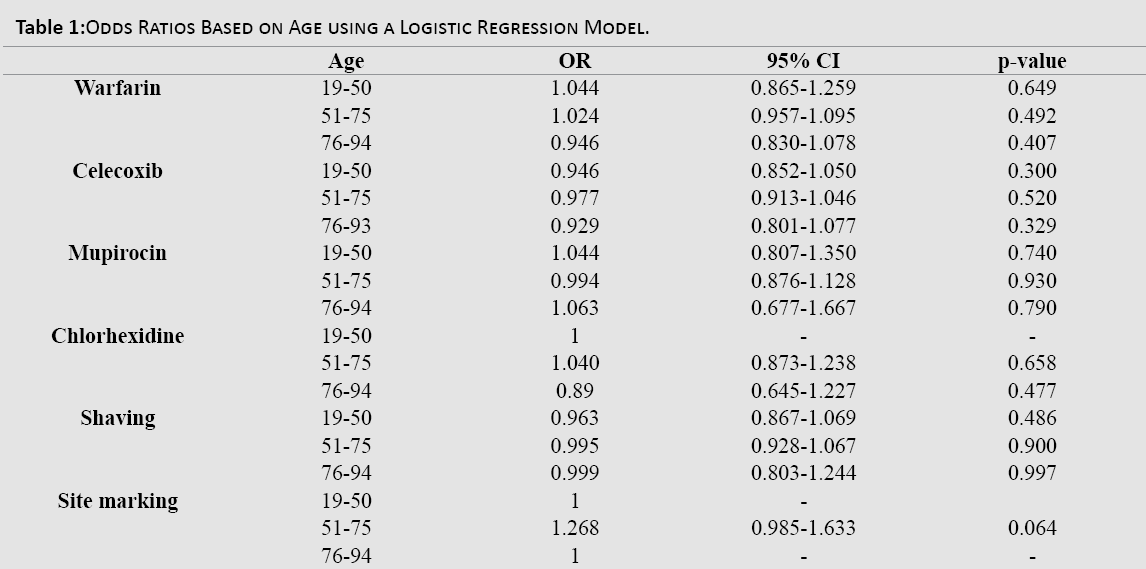
On sub-group analysis based on age, odds ratios with a 95% confidence interval were calculated for each medication category using a logistic regression model. The compliance odds ratio based on age groups of 19-50, 51-75, and 76-94 years for each point of the protocol did not produce any statistically significant results (Table 1).
The age sub-group had further breakdown for average days of use of a medication based on their age. In regards to the average number of days celecoxib was taken the <50 year-old age group averaged 2 days (SD 1.06 days), the 51-75 year-old age group averaged 1.90 days (SD 0.55 days), and the 76-94 year-old age group averaged 1.76 days (SD 0.64 days) (Figure 2). In regards to the average number of days mupirocin was used, the <50 year-old age group averaged 4.71 days (SD 2.90 days), the 51-75 year-old age group averaged 3.92 days (SD 2.34 days), the 76-94 year-old age group averaged 4.29 days (SD 2.75 days) (Figure3). Finally, in regards to the average number of days chlorhexidine was used the <50 year-old age group averaged 2.69 days (SD 0.63 days), the 51-75 year-old age group averaged 2.75 days (SD 0.83 days), the 76-94 yearold age group averaged 2.84 days (SD 0.81 days) (Figure 4).
In sub-group analysis based on gender for warfarin, celecoxib, mupirocin, chlorhexidine, shaving, and site marking compliance no difference was found between genders in all categories with all measurements failing to meet statistical significance (Table 2).

The prevailing definition of compliance/adherence is “the extent to which a person’s behavior (in terms of taking medication, following diets, or executing lifestyle changes) coincides with medical or health advice.” In our study we found that the percentage of compliant patients with the preoperative regimen set forth by our Joint Replacement Surgical Pathway was acceptably high with the lowest overall percent being no lower than 84.41% for overall mupirocin compliance. This is not to say that our method is not open to improvement to help patients fulfill their preoperative responsibilities, but with no studies, to our knowledge, on preoperative total joint regimen adherence, our study may help give physicians a tool to use for the betterment of patient preoperative compliance.
Other studies have been reported in the literature in regards to preoperative compliance. Shirakawa et al. had an 82.6 percent preoperative compliance with preoperative nutrition optimization protocols. They found that the patients who were compliant with their preoperative nutrition protocol had a significant lower frequency of wound infection.[7] This type of study demonstrates how high percentage patient compliance can lead to good outcomes. However, other studies show lower levels of patient compliance. Chiu et al., demonstrated a suboptimal compliance in their study of osteoporosis regimens. In their study only 52.3% of males at one year and 37.5% of males at two years had good compliance with their osteoporosis regimens.[2]
We were able to obtain a higher patient compliance than previous studies in the literature that report patient compliance.[2,3,5] A study similar to ours in nature that focused on patient education was conducted by Collins et al. They measured compliance of women pledging to use contraception while on isotretinoin. Their highest percentage of compliance for using 2 forms of contraception was 79%.[3] Our study suggests it is reasonable for a surgeon to expect a patient, with the proper preoperative education, to adhere to a preoperative regimen and that the patient can be involved with his preoperative management based on our compliance rates.
The study had a few limitations. First, this study was an observational study. There was no control group prior to the introduction of the joint replacement surgical home. However, the intent of the study was not to compare the results of an intervention, but instead this study’s goal was to demonstrate the current protocols being used are successful in helping patients be compliant with preoperative instructions. This was demonstrated with obtaining a compliance rate that is comparable, if not higher than other studies' compliance percentages.[2,7] The protocols we used included 1) a nurse practitioner (NP) in clinic educating the patient before surgery, during the preoperative visit, about the medications the patient should be taking, 2) the prescriptions for the preoperative medications were given by the NP during that visit, and 3) patients were instructed to come to a two-hour presurgical education class, which was offered two times a month, discussing medications and the surgery that was to be performed. The class agenda consisted of details on what would happen during the preoperative, operative, and postoperative stages of the surgery and hospital stay. It also provided the patients instruction on how to prepare during the days leading up to the surgery, including reminders to carry out the preoperative treatment regimen, as well as reviewing what the recovery process would look like in regards to physical rehab. Secondarily, this study was limited by having only one surgeon and one institution involved. A different area of the country or a different surgeon educating his/her patients differently might have different results than what was seen in this study. However, the information contained in this study could be helpful in setting the groundwork for other institutions to consider starting an educational session for their practices in order to obtain higher preoperative compliance and patient engagement in their care. Also, the study population had a higher prevalence of females. This could also affect the rate of compliance because it is unknown if females are more or less compliant in preoperative regimens when compared to men. The evaluation was also limited in its scope of attaining information as to ‘why’ a patient was not compliant. The study’s goals were not to obtain ‘why’ patients were non-compliant with the preoperative regimen, but instead to answer the first question of ‘were’ they compliant. Further studies could be designed to study as to why patients were not compliant with preoperative regimens.
In conclusion, compliance with preoperative guidelines such as medications and body washes are actions a patient can take in order to improve outcomes and decrease the risk of adverse events after total joint arthroplasty. The current regimen of preoperative preparation has no lower than 84.41% compliance rate and is an equivalent rate if not higher than other studies looking at patient compliance rates. While more steps can be taken to increase the rate of compliance among total joint arthroplasty patients, this study demonstrates that the current regimen is a solid starting point for instituting patient preoperative preparation in total joint arthroplasty, and that age or gender has a minimal effect on compliance with preoperative preparation.