Case Report - (2016) Volume 17, Issue 3
Shuhei Oana, Sho Shibata and Takayuki Matsumoto
Division of Gastroenterology, Department of Internal Medicine, School of Medicine, Iwate Medical University, Morioka City, Japan
*Corresponding Author:
Shuhei Oana
Uchimaru 19-1, Morioka City
Iwate 020-8505, Japan
Phone: +81-19-651-5111, ext 2314
Fax: +81-19-652-6664
E-mail: shuoana@iwate-med.ac.jp
Received: February 8th, 2016; Accepted: March 14th, 2016
Endoscopic ultrasound-guided cyst drainage is increasingly performed in the treatment of pancreatic pseudocysts and walled-off pancreatic necrosis. We have experienced three cases of colonic fistula after endoscopic ultrasound-guided cyst drainage. Case #1 was a patient with walled-off pancreatic necrosis, in which a fistula into the transverse colon occurred as a late complication of endoscopic ultrasound-guided cyst drainage. We closed the fistula with fibrin glue and endoscopic clips. Case #2 was a patient with walled-off pancreatic necrosis treated by endoscopic ultrasound-guided cyst drainage. During repeat endoscopic ultrasoundguided cyst drainage, a fistula was noted at the splenic flexure of the transverse colon, which was confirmed by contrast enema. He was successfully treated by diverting colostomy and abscess drainage. Case #3 was a patient with a history of pylorus-preserving pancreaticoduodenectomy for intraductal papillary-mucinous neoplasm. He presented with a pancreatic pseudocyst in the pancreatic tail. During endoscopic ultrasound-guided cyst drainage, a fistula was noted in the transverse colon. The patient was treated by naso-biliary tube with a pig-tail biliary stent and total parenteral nutrition. Our experience suggests that colonic fistula after endoscopic ultrasound-guided cyst drainagecan be managed conservatively.
Keywords
Pancreatic Pseudocyst
Abbreviations
CT computed tomography; EUS endoscopic ultrasonography; EUS-CD endoscopic ultrasound-guided cyst drainage; OTSC over-the-scope clip; PCF pancreatic colonic fistula; PPC pancreatic pseudocyst; WOPN walled-off pancreatic necrosis
INTRODUCTION
It has been reported that pancreatico-colonic fistula (PCF) occurs spontaneously in 0.4 ~ 5.0% of patients with acute pancreatitis [1, 2]. The fistulous tract is usually suspected with a small amount of air in the pancreatic pseudocyst (PPC) on computed tomography (CT). In cases of uncontrolled infection in patients with walled-off pancreatic necrosis (WOPN) and pancreatic pseudocysts (PPC), contrast radiography may depict a PCF. PCF is usually refractory to medical management and historically requires prompt surgical treatment. However, there have been cases of PCF successfully treated by non-surgical interventions [3, 4]. We present herein three cases of PCF after endoscopic ultrasound-guided cyst drainage (EUS-CD). The cases were treated by surgical or endoscopic intervention with conservative therapy.
CASE REPORTS
Case #1
A Sixty-five-year-old woman was admitted to our hospital with upper abdominal pain. The patient was diagnosed with grade E acute pancreatitis by abdominal computed tomography (CT), with four poor prognostic factors based on Ranson’s criteria. We treated her conservatively with intravenous fluid resuscitation, protease inhibitors, antibiotics, and immunoglobulin (Figure 1a-d). While the patient initially recovered, laboratory data on hospital day 26 revealed signs of inflammation and infection including leukocytosis (12,000 cells/μL) and an increase in C-reactive protein (CRP) (178 mg/L). CT findings on hospital day 40 demonstrated a large area of walled-off pancreatic necrosis (WOPN) anterior to the pancreas (Figure 2a). We treated the patient by EUS-CD for drainage and placed a naso-biliary tube and a pig-tail biliary stent into the PPC.
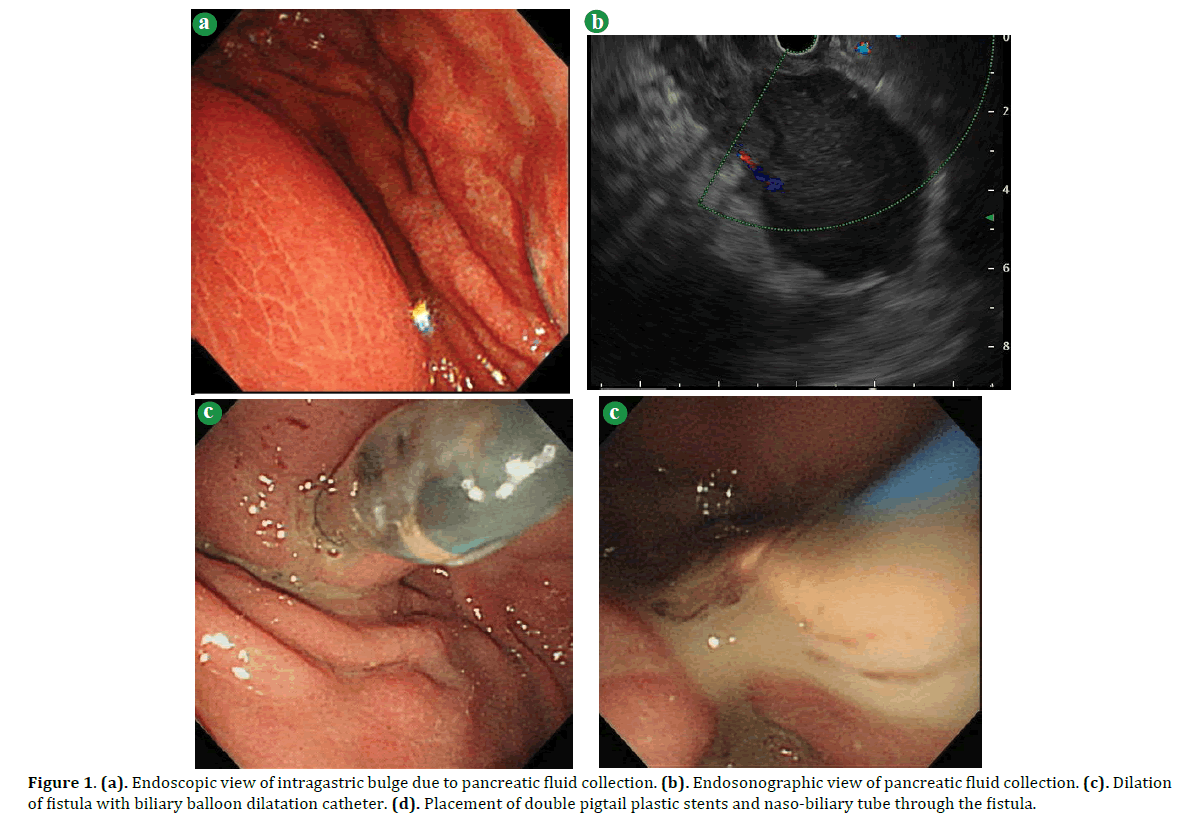
Figure 1. (a). Endoscopic view of intragastric bulge due to pancreatic fluid collection. (b). Endosonographic view of pancreatic fluid collection. (c). Dilation of fistula with biliary balloon dilatation catheter. (d). Placement of double pigtail plastic stents and naso-biliary tube through the fistula.
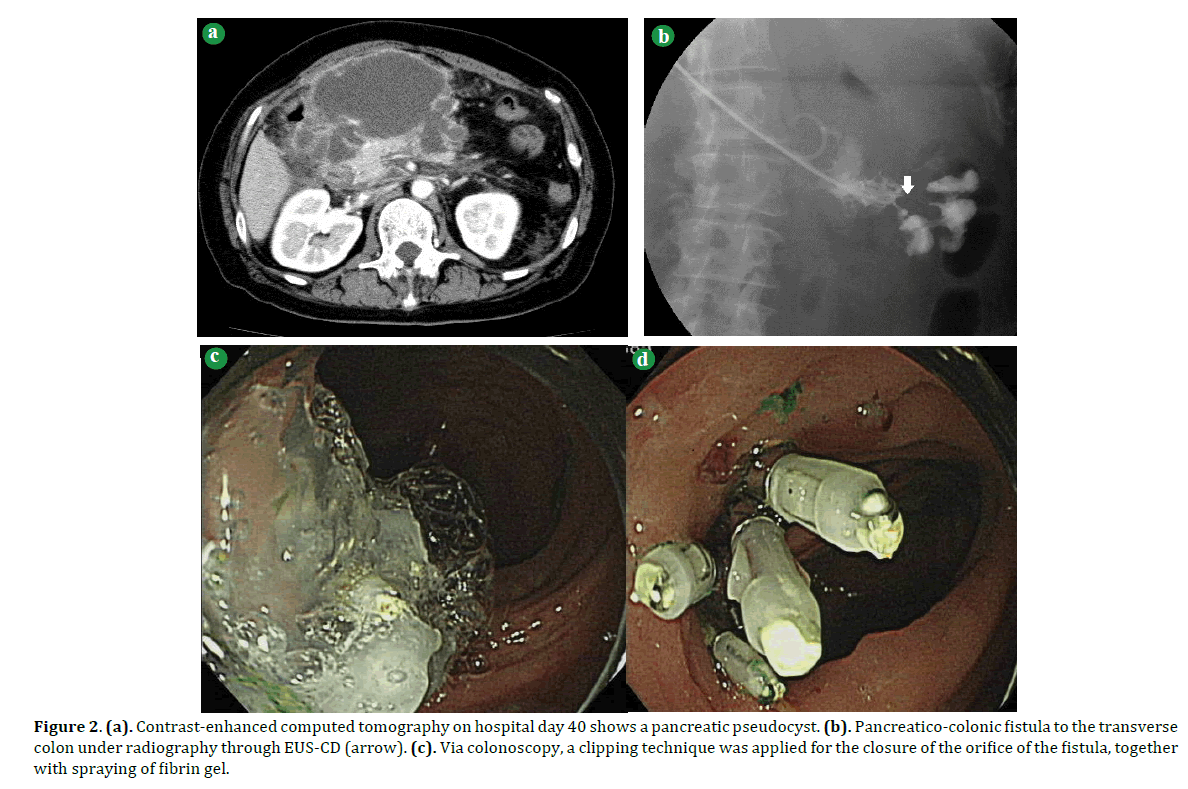
Figure 2. (a). Contrast-enhanced computed tomography on hospital day 40 shows a pancreatic pseudocyst. (b). Pancreatico-colonic fistula to the transverse colon under radiography through EUS-CD (arrow). (c). Via colonoscopy, a clipping technique was applied for the closure of the orifice of the fistula, together with spraying of fibrin gel.
The procedure for EUS-CD was as follows. 1) EUS using linear array type was performed to look for the site with an optimal point at the gastric and cystic walls. 2) The WOPN was punctured by a 19G EUS-FNA needle. 3) A guide-wire was introduced through the needle. 4) A 5.5 Fr tapered tip cannula was passed over the guide-wire to puncture the bowel wall and cyst wall. 5) The fistulous track was dilated with either a 6 or 8 mm biliary balloon dilatation catheter over the wire under endoscopic or EUS view. 6) After dilatation, a 6 F double lumen endoscopic cannula was passed over the guide-wire into the pseudocyst wall. 7) One or two 7.2 Fr double pigtail stents and a naso-biliary drainage tube are inserted over the wire and placed through the fistula, thereby communicating with the pseudocyst.
Daily irrigation with saline solution was initiated through the naso-cystic tube. However, contrast radiography through the naso-cystic drainage tube on the 54th hospital day showed a colonic fistula into the transverse colon (Figure 2b). With the use of contrast medium with indocyanine green through the naso-cystic drainage tube, we could identify the orifice of the fistula colonoscopically and close the orifice with endoscopic clips and fibrin gel (Figure 2c). Subsequently, another orifice was identified at the hepatic flexure of the transverse colon by radiography through a naso-biliary drainage tube, but colonoscopy failed to identify the orifice. We thus chose to insert an endoscope directly into the PPC and spray fibrin gel inside the PPC. Thereafter, the PPC gradually decreased in size. The patient was initiated on oral food intake on hospital day 69 and discharged on hospital day 101. She has been doing well without any recurrence of the PCF during our four-year follow up.
Case #2
A Thirty-four-year-old man was referred to our hospital for the treatment of infected WOPN. Abdominal CT on admission showed WOPN with a fluid collection in the lesser sac (Figure 3a). Laboratory data revealed leukocytosis (12460/μL), an elevated amylase (430 IU/dL), and an increase in the CRP (27.4mg/L). We immediately performed EUS-CD for drainage and placed a naso-biliary tube and a pig-tail biliary stent into the WOPN. As applied to Case #1, we carried out EUS-CD for the patient. During repeat EUS-CD on the 7th day, radiography through the naso-biliary drainage tube revealed a fistula at the splenic flexure of the transverse colon (Figure 3b). We performed colonoscopy and a contrast enema, which clearly showed a fistula at the transverse colon and pooling contrast in the WOPN (Figure 3c). We did not choose to close the fistulous tract since the lower part of the cavity extended to the pelvis and contained air (Figure 3d). Because it was presumed that the uncontrolled infection of WOPN was due to this fistula (Figure 3e), he was treated surgically with a diverting colostomy and abscess drainage. After the surgery, his condition improved and he was discharged on the 37th day.
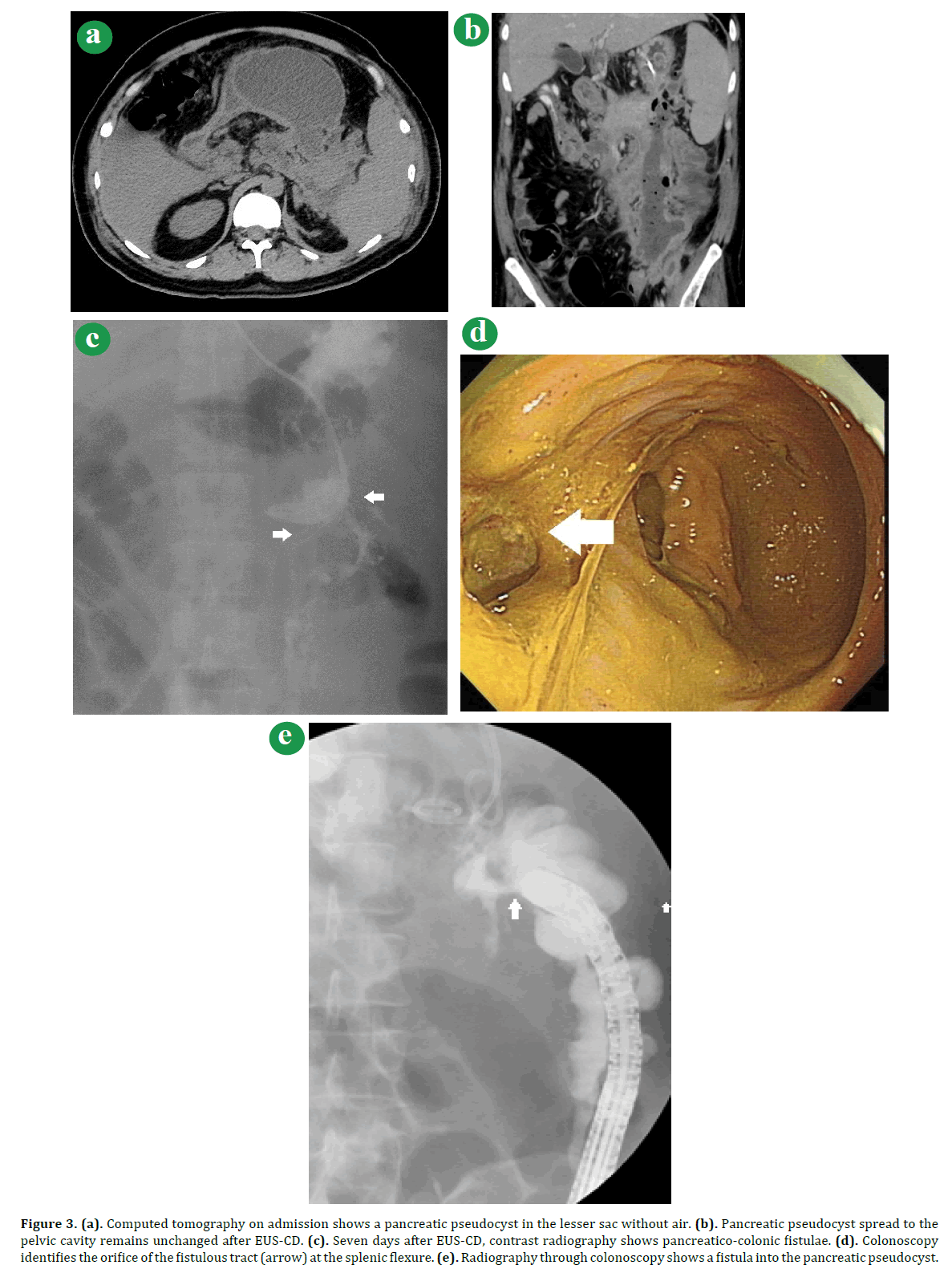
Figure 3. (a). Computed tomography on admission shows a pancreatic pseudocyst in the lesser sac without air. (b). Pancreatic pseudocyst spread to the pelvic cavity remains unchanged after EUS-CD. (c). Seven days after EUS-CD, contrast radiography shows pancreatico-colonic fistulae. (d). Colonoscopy identifies the orifice of the fistulous tract (arrow) at the splenic flexure. (e). Radiography through colonoscopy shows a fistula into the pancreatic pseudocyst.
Case #3
A Seventy-six-year-old man with a prior history of pylorus-preserving pancreaticoduodenectomy for intraductal papillary-mucinous neoplasm thirteen years previously was referred to our hospital for pyrexia. Abdominal CT on the first visit showed a PPC extending from the pan`creatic tail to the lesser sac (Figure 4a). Laboratory tests showed an increase in CRP (12.7 mg/dL). Abdominal CT did not show any air in the PPC (Figure 4b). For drainage, we treated the patient by EUS-CD and placed a naso-biliary tube and a pig-tail biliary stent into the pancreatic abscess. During the intervention, we observed a fistula at the transverse colon. On the 14th day after the EUS-CD, the patient recovered without any signs of infection. Colonoscopy performed two weeks later together with naso-biliary drainage tube radiography with indocyanine green did not show any sign of fistula in the transverse colon (Figure 4c). With repeated lavage through the drainage tube, the purulent discharge diminished and the patient’s symptoms improved. On the 49th hospital day, oral intake was resumed. He was subsequently discharged in good health without any symptoms.
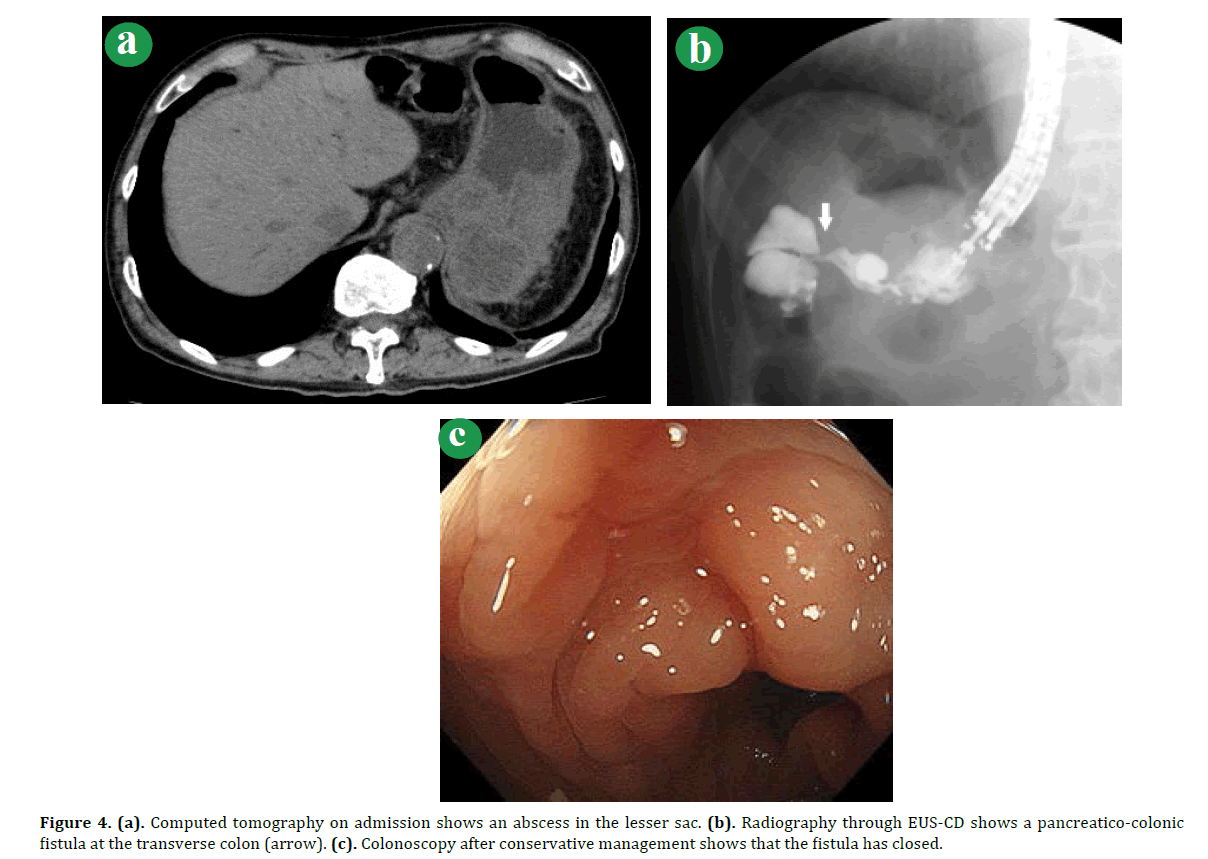
Figure 4. (a). Computed tomography on admission shows an abscess in the lesser sac. (b). Radiography through EUS-CD shows a pancreatico-colonic fistula at the transverse colon (arrow). (c). Colonoscopy after conservative management shows that the fistula has closed.
DISCUSSION
PPC can occur after severe pancreatitis and disappears within 6 weeks in most cases. In rare cases, however, a pancreatic colonic fistula (PCF) occurs as a complication of PPC after acute necrotizing pancreatitis. It has been reported that the median time from the diagnosis of pancreatitis until diagnosis of PCF is 89 days [5].
Pancreatico-gastrointestinal fistulae usually act as a good drainage route for infected PPC if the fistula is not to the colon. While the incidence of PCF is much lower than fistulae into the stomach or small bowel, a fistula to the colon is a serious problem that can occur as a sequela of PPC or as a complication of the management of PPC. Furthermore, PCF are difficult to diagnose and carry an increase in mortality. As summarized in Table 1, the clinical outcomes after surgery for PCF are fair to poor, with a mortality rate ranging from 0% to 67%. PCF has been shown to be most commonly associated with WOPN or PPC; they rarely occur spontaneously. Typically, the clinical manifestations of PCF include diarrhea, hematochezia, and fever. Less frequently, however, PCF may result in a large bowel obstruction, which accounts for less than 1% of patients with PCF [1, 2, 6]. In patients with sepsis and hemorrhagic transformation, conservative treatment including bowel rest and antibiotic administration has been shown to result in 50% mortality [7, 8]. Such a high mortality seems to be a consequence of difficulty in the diagnosis of PCF, since it cannot typically be found unless percutaneous catheter placement for drainage of WOPN or PPC is performed with subsequent drainage tube radiography showing flow of contrast into the colon [9]. The underlying mechanism for spontaneous fistula formation is thought to be an elevation in pressure due to fluid accumulation within PPC and WOPN. The proteolytic enzymes within the fluid then invade adjacent organs or vessels inducing ischemic changes resulting in penetration of the walls of the most vulnerable organs, thereby forming a fistula [10]. The PCF in our cases was found during or after EUS-CD for PPC or WOPN. It thus seems possible that an increase in the luminal pressure within the cystic space by saline infusion was associated with fistula formation in our cases.
WOPN or PCC with PCF has been shown to result in high mortality if left untreated surgically [11, 12]. However, there have been recent reports of several cases of PCF successfully treated by endoscopic interventions, such as pancreatic stent, [8] endoscopic clip with fibrin glue,[3, 4] and over-the -scope clip (OTSC) system (Table 1). While Renteln et al. [13] reported upon the efficacy of conventional endoscopic clips for the closure of small perforations in PPC, endoscopic clips may not be sufficient for the closure of larger perforations [14]. In this regard, OTSC may be a promising technique for the closure of larger PFC [15]. In contrast, Kwon et al. [16] reported the efficacy of the use of bowel cleansing by polyethylene glycol for the management and closure of PCF.

The management as described here was variable, with a purely surgical intervention in one case, an endoscopic intervention in another case, and a purely conservative management in the remaining case. Our experiences suggest that maintenance of a clean colon was valuable for the management of PPC with PCF in cases of a narrow fistulous tract between the PPC and the colon.
We chose a surgical intervention for Case 2, which was accompanied by uncontrollable infection in a PPC secondary to PCF. A similar case of an infected PCF has been described in the literature [8]. Although the indications for endoscopic or conservative managements for patients with PCF remain to be established, our cases seem to suggest that PCF with a controlled infection may be managed with EUS-CD together with conservative or endoscopic therapy. The management strategy may depend on the size of the fistula and the grade of regurgitation of the colonic content into the fistulous tract and the PPC or WOPN.
Currently, there are no established guidelines for the treatment of PPC and WOPN with colonic fistula. Our cases suggested that unlike percutaneous drainage EUS-CD can avoid local complications relating to the drainage. In addition, drainages under EUS-CD can be applied directly to the fluid cavity of PPC and WOPN. It thus seems possible that EUS-CD may be an alternative procedure for the treatment of complicated PPC and WOPN. Our case suggested that large-scaled clinical trials with the use of EUS-CD should be seriously considered for the treatment of complicated PPC and WOPN.
Conflicts of interest
The authors do not have any conflicts of interest to disclose.