- (2009) Volume 10, Issue 4
Norman Oneil Machado, Pradeep Chopra
Department of Surgery, Sultan Qaboos University Hospital. Muscat, Oman
Received February 18th, 2009 - Accepted May 6th, 2009
Context Metastatic cancer to the pancreas is rare and accounts for less than 2% of all pancreatic malignancies, metastasis from renal cell carcinoma being predominant. While symptomatic patients present with obstructive jaundice, abdominal pain, or GI bleeding, the diagnosis is often made in asymptomatic patients during follow-up for renal cell carcinoma. Hence, a high index of clinical suspicion is required in a patient who presents with a pancreatic tumor following a nephrectomy for renal cell carcinoma. Case report We report the case of a patient in whom a lesion was detected in the head of the pancreas, following a nephrectomy performed 5 years previously for renal cell carcinoma. A magnetic resonance scan revealed a well-defined lesion in the head of the pancreas. The patient underwent a pancreaticoduodenectomy and histopathology confirmed a metastatic renal cell carcinoma. Two years after the surgery, the patient is doing well. The literature is reviewed for pancreatic metastasis from renal cell carcinoma, for metastatic pattern, surgical management and outcome. Conclusion Pancreatic metastases are usually detected during the follow-up of patients having undergone a previous nephrectomy for renal cell carcinoma. Typically, the interval between a nephrectomy and pancreatic metastasis is long. The literature contained more than 250 cases of pancreatic resection for metastatic renal cell carcinoma. The median duration of presentation was 10.5±6.5 years following a nephrectomy. The lesions are multifocal (3.2±1.5) in about 39% of patients and resectable in 80%. A high resectability rate is characteristic of metastasis from renal cell carcinoma as compared to primary pancreatic cancer. The five year survival rate is between 43 and 88%.
Carcinoma, Renal Cell; Neoplasm Metastasis; Pancreatic Neoplasms; Pancreaticoduodenectomy
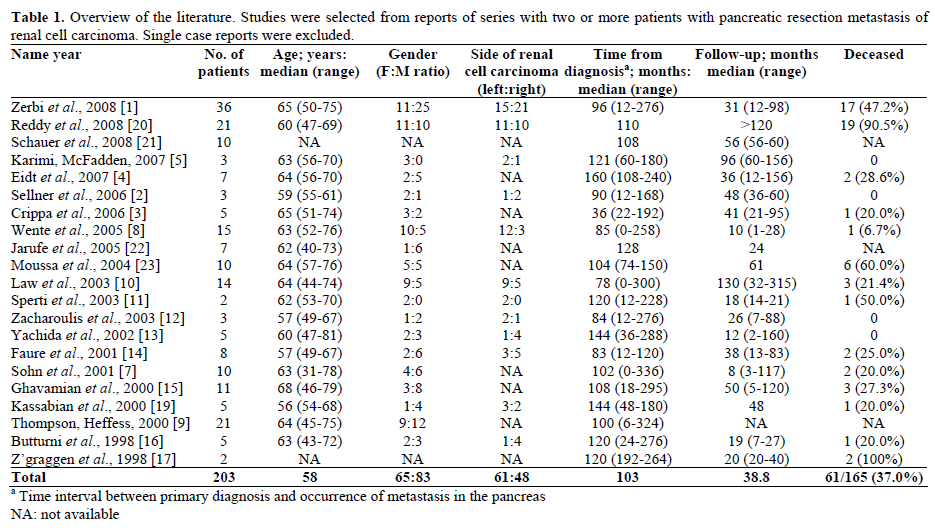
Metastatic cancer to the pancreas from another primary site is rare [1, 2]. Renal cell cancer, along with malignant melanoma, and lung, colon and breast carcinoma, are among the few tumors known to metastasize to the pancreas [3, 4]. Pancreatic metastases from renal cell carcinoma present synchronously with widespread metastatic disease in 12% of cases and therefore surgical resection may not be favorable [1, 2]. However, there have been numerous case reports and case series of isolated metachronous pancreatic or peripancreatic metastasis from a renal primary lesion treated with surgical resection [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16., 17, 18, 19, 20, 21, 22, 23, 24, 25] (Table 1). The overall outcome of a solitary metastasis in the pancreas treated with resection is promising with the five year survival rate ranging from 43 to 88% [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18].
A 64-year-old man had undergone a left radical nephrectomy for renal cell carcinoma five years previously. The renal tumor measured 11x10 cm and was found in the upper pole. The tumor was classified as grade 3 in the Furham nuclear grading system (large clear granular cells with nuclei showing obvious pleomorphism and enlargement). There was vascular involvement, and large hilar veins also showed tumor invasion. No capsular involvement was seen and the ureter and hilar lymph nodes were tumor free. However, the tumor also involved the calyx. Staging investigations, including a CT scan, revealed no obvious metastasis. A bone scan was not carried out. The tumor was staged as T3b, N0, M0. The patient did not receive any postoperative chemotherapy but was monitored with regular CT scans.
He remained well until recently when he was found to be jaundiced. On examination, he was well preserved and deeply jaundiced. The abdomen was unremarkable except for a well healed left nephrectomy scar; an ultrasound scan of the abdomen showed a mass in the head of the pancreas. Additional evaluations were carried out. Liver function tests were abnormal indicating obstructive jaundice (ALP, 369 IU/L; reference range: 40-129 IU/L; total bilirubin, 93 mmol/L; reference range: 0-17 mmol/L). The preoperative CA 19-9 levels were within normal limits. An MR cholangiogram revealed a well defined 4 cm mass localized in the pancreatic head with obstruction of both the common bile duct and the pancreatic duct (Figure 1). Staging investigations revealed no secondary neoplasias. A diagnosis of obstructive jaundice secondary to carcinoma of the head of the pancreas along with a possibility of renal metastasis was considered. He underwent surgery and a laparotomy revealed a 4 cm lesion in the pancreatic head with a dilated biliary system. There was no regional lymphadenopathy. A classical Whipple’s pancreaticoduodenectomy was performed. He did well until the 7th postoperative day when he developed a significant upper GI bleed. An upper GI endoscopy revealed clots in the stomach although a definite bleeding site was not detected. The bleeding persisted for 48 hours which necessitated a re-laparotomy. No active bleeding was noted and all the anastomoses were intact however it was decided to reinforce them. However, he continued to have a slow persistent upper GI bleed with a drop of 1.5 g/dL of hemoglobin per day. He was then treated with two doses of activated recombinant factor VII infusion at a dose of 50 μg/kg/h to which he responded. His bleeding stopped and he made steady progress. He was discharged seven days later.
Histology showed a circumscribed encapsulated 5x3x3 cm lesion in the pancreatic head. The lesion was composed of solid sheets of cells divided by bands of fibrovascular tissue into large nests and alveoli. The cells showed moderate clear to granular eosinophilic cytoplasm with well-defined cell borders. The nuclei were central and pleomorphic with conspicuous nucleoli. The lesion was surrounded by a thick collagenous capsule and completely separated from the pancreatic tissue with no infiltration (Figure 2). The morphology was identical to that of the primary renal tumor which was reviewed (Figure 3). A final diagnosis of metastatic clear cell renal cell carcinoma of the head of the pancreas was reached. The surgical margins were free of tumor. He has remained well for about 2 years (his last follow-up). A recent bone scan carried out for generalized bone pain, showed bone metastasis. He is being managed by an oncologist and remains generally well except for the bone pain
The pancreas is a rare site of solitary metastasis but it is often involved in diffuse metastatic disease [1, 2, 3, 4, 8, 9]. The behavior of renal cell carcinoma metastasis is unusual and not well understood [1, 2, 3, 4]. In a large published series, metastasis from primary renal cell carcinoma to the pancreas made up from 0.25 to 3% of all resected pancreatic specimens [1, 9, 14]. However, there is a bias towards late occurring single isolated metastasis and the incidence reported may not be representative of the frequency of metastasis to the pancreas by different malignancies. Addressing this issue, in a study comparing the incidence of pancreatic metastasis among 4,955 adult autoptic specimens with 973 surgical specimens, the incidence of pancreatic metastasis in the autoptic specimens was 3.83% (190 cases) among which metastasis from renal cell carcinoma was 0.08% (4 cases). In contrast, even though the incidence of pancreatic metastasis in 973 resected specimens was similar (3.93%; 38 cases), the incidence of metastasis from renal cell carcinoma was 0.61% [26]. However, renal cell carcinoma was the most common primary tumor leading to solitary pancreatic metastasis among the resected specimens [3, 4]. Metastases often present many years after a nephrectomy [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. Metastasis to the pancreas may be present at the time of diagnosing the primary renal cell carcinoma often as part of an extensive concomitant metastasis or it may be found at the follow-up of patients operated on for renal cell carcinoma.
These patients could be asymptomatic. In a review of 236 cases in the literature, Sellner et al. [2] reported 35% of these patients to be asymptomatic with others presenting with symptoms which included abdominal pain (20%), GI bleeding due to duodenal infiltration (20%), obstructive jaundice (9%), weight loss (9%), and pancreatitis and diabetes (3% each). The median tumor diameter in the asymptomatic subgroup was reported to be 25 mm (range: 14-48 mm) as compared to 45 mm in symptomatic group [6]
The mode of spread of renal cell carcinoma to the pancreas is controversial and can either be hematogenous or via lymphatics with direct spread to the pancreas being unusual. Spread through lymphatics may occur by retrograde lymph flow secondary to tumor infiltration of the retroperitoneal lymph nodes [18]. Hematogenous spread may occur along the draining collateral vein of a hypervascular renal tumor with or without associated renal vein thrombosis [18]. However, analysis by Sellner et al. [2] of the pattern of spread in 236 cases has revealed the diffuse distribution of solitary pancreatic metastasis. They occurred in all parts of the pancreas irrespective of whether the renal cell carcinoma was from the right or the left kidney. The frequency of multiple metastases and infrequent metastasis to the lungs and other organs was also noted. They felt that the invasion of normal and abnormal lymphatic and venous communication between renal cell carcinoma and the pancreas does not play an important role. All this fails to explain the lack of a relationship between the site of isolated pancreatic metastasis and the site of primary renal cell carcinoma in the right or left kidney [2]. This observation argues in favor of hematogenous systemic spread but does not explain the discrepancy between the relative frequency of multiple isolated pancreatic metastases and the absence of metastasis to other organs [2]. The most likely explanation proposed by them for this unique behavior was the inherent special biology of the tumor. They hypothesized that the tumor cells had a high affinity for the parenchyma of the pancreas which is supported by the finding of late metachronous metastasis in the residual pancreas [2].
The preoperative diagnosis of pancreatic metastasis is a suspicion based on the fact that the patients had a previous resection for a renal cell carcinoma. Abdominal ultrasonography and CT scan are reliable although the typically hypervascular image seen in CT could resemble that of an endocrine pancreatic tumor as primary pancreatic tumors tend to be hypovascular [19, 20, 21]. Magnetic resonance imaging is now being increasingly used in the diagnosis of pancreatic disease as it was in our case [20]. Endoscopic ultrasound can also be used for imaging; lesions are typically rounded well-delineated masses which are hypoechoic in comparison to adjacent pancreatic tissue [21]. However, in view of the diagnostic difficulties in individual cases, many authors now recommend establishing tissue diagnosis with a fine needle aspiration biopsy which could be guided either by ultrasound, CT or endoscopic ultrasonography. Endoscopic ultrasonography-assisted fine needle aspiration cytology has been reported as a safe and minimally invasive method for diagnosing non-primary pancreatic neoplasms. An accurate diagnosis of the primary lesion is further facilitated by careful evaluation of the clinical history, cytomorphology and ancillary techniques especially those applied to cell block material including immunohistochemistry [27]. The above investigations facilitate definitive tissue diagnosis and establish an assessment of resectability [1, 2, 27, 28, 29, 30].
Surgical resection of primary renal cell carcinoma and metastatic deposits remain the most effective treatment since chemotherapy, radiotherapy and hormonal therapy have generally proved ineffective [1, 8, 9, 18]. Resection of a pancreatic metastasis may involve a standard pancreaticoduodenectomy or a distal pancreatectomy depending on the location of the secondary deposit. There has also been a report of successful combined resection of the pancreas and inferior vena cava when the inferior vena cava was involved by the metastatic tumor [25]. Atypical resection of pancreatic metastasis from renal cell carcinoma, such as duodenum-preserving pancreatic head resection, middle pancreatectomy and enucleation of the tumor, has been adopted by some authors [1, 6]. Atypical resection has been adopted based on the fact that these lesions are well-encapsulated. A relatively good prognosis has been observed which may be due to the absence of lymph node spread and local recurrence [1, 6]. Bassi et al. [6], adopting a policy of atypical resection (duodenum-preserving pancreatic head resection), observed a 29% rate of pancreatic recurrence (5 out of 17 cases). Of the five recurrences, two occurred after a standard resection (distal pancreatectomy) and three after atypical resection (duodenum-preserving pancreatic head resection); hence, a radical resection was recommended. However, there are others who do not share this view and feel that, since the frequency of multiple metastases is often more than 2 (average 3.2), the occurrence of pancreatic recurrence is likely to be an expression of an undetected multifocality rather than the consequence of an inadequate surgical procedure [1]. The choice of a standard or an atypical surgical procedure is then probably less important than a careful search for multiple metastases [1]. Intraoperative pancreatic metastases are detected by a combination of complete mobilization of the whole pancreas, careful manual palpation and intraoperative ultrasonography searching for multiple metastases [1, 2]. Intraoperative ultrasonography increases the accuracy of detecting pancreatic nodules and precisely defines the relationship between the nodule and the pancreatic duct [1, 13]. When pancreatic metastasis is detected, the choice of performing a standard or an atypical resection is probably not important if the margin of resection is cancer free. The advantage of a standard resection is that it enables the removal of peripancreatic lymph nodes. However, an extensive review of the literature indicates the involvement of lymph nodes in metastatic pancreatic malignancy to be extremely unusual [1, 2]. Therefore, they suggest an individual surgical approach with optimal resection strategy which achieves adequate disease-free resection margins and maximal tissue preservation of the pancreas [1]. Multifocality of pancreatic metastasis has been reported to be in the range of 20-45% [1, 2, 6, 8, 9]. In one report, a preoperative multifocality detection of 17.4% increased to 34.8% on pathological examination of the resected specimen [1]. Therefore, this discrepancy suggests that multiple metastases are more frequent than usually believed.
There are several reports suggesting that previously resected renal cell carcinoma metastasis at other sites including the thyroid, adrenal glands and lung should not discourage aggressive treatment of a pancreatic secondary metastasis once it is confirmed to be the only site of recurrent disease [1, 2, 6, 8]. Surgical treatment of isolated pancreatic metastasis from neoplasms other than renal cell carcinoma carries a poor prognosis as they signal the onset of disseminated metastatic disease [2, 3, 4]. By contrast, the outcome of surgery for isolated pancreatic metastasis from renal cell carcinoma is clearly superior with a mean survival of 4 years and an actuarial 5-year survival ranging from 43 to 88% which is even better than that of primary adenocarcinoma of the pancreas [1, 2] (Table 1). The positive outcome of pancreatic metastasis from renal cell carcinoma suggests that it should not be regarded as an accidental initial manifestation of impending diffuse metastatic disease and that it is important to correctly diagnose isolated pancreatic metastasis from renal cell carcinoma and subject them to radical resection [1, 2, 8, 9]. Factors associated with a favorable prognosis include a long disease-free interval after resection of the primary tumor, a single metastatic deposit with central necrosis and complete excision of the secondary deposit with histologically negative margins [18]. Others have reported that the tumor grade of the pancreatic metastasis correlated with the grade of the primary renal cell cancer and that tumor grade was a predictor of survival with a median survival of 41 months for grade 2 cancer and 10 months for grade 3 cancer [24].
When metastases are limited to the pancreas, complete resection can provide a 5-year survival rate of from 43 to 88% [1, 2, 7, 9, 10]. The overall 24- and 60-month survival probabilities are reported to be 0.84 and 0.53, respectively in patients who underwent resection [6]. Corresponding values of survival probability at 24 and 60 months in patients treated conservatively were 0.53 and 0.26, and the difference between the 2 groups was significant (P=0.040) [6]. Radical resection was omitted in only a few exceptional cases [1, 2]. When the survival data of 10 patients with non resected disease was compared with 139 patients who underwent radical resection, the actuarial 3- and 5-year survival rates of 21% and 0% for a non-resected metastasis were significantly poorer (P=0.383) than those for resected lesions (78% and 72%, respectively) [2]. Analyzing 11 studies with 5 or more patients which addressed pancreatic metastasis from renal cell carcinoma and which included long term survival, Reddy et al. noted that, in seven studies (64%), the five-year survival rate was 80% from the time of the pancreatic metastatectomy. A Kaplan Meir analysis done on 112 patients yielded a median survival rate of 8.75 years (range: from 0.6 months to 18.5 years) and 5-year survival rate of 66% [24].
Immunoreactive cytokines have been the mainstay of treatment of metastatic renal cell carcinoma for the last 15 years and other areas of development of immunotherapy are now represented by a cancer vaccine and allogenic hemopoietic stem cell transplantation [31]. However, immunoreactive cytokine results have been disappointing and have not led to better survival rates in most studies [32]. Several antiangiogenetic agents, such as bevacizumab, sunitinib and sorafenib, have showed promising activity in this disease [31]. In a randomized double blind placebo controlled trial, sorafenib, a multikinase inhibitor of tumor proliferation and angiogenesis, was analyzed in patients with advanced clear cell renal cell carcinoma [33]. The study concluded that, compared to a placebo, treatment with sorafenib (400 mg bid) prolonged progression free survival in patients with advanced clear cell renal cell carcinoma (best response 10% in patients receiving sorafenib as compared to 2% in those receiving placebo: P<0.001). However, treatment is associated with increased toxic effects including diarrhea, rash, fatigue, hand and foot skin reaction and occasionally hypertension and cardiac ischemia [33]. Surgical resection for pancreatic metastasis is usually combined with other treatment approaches in different periods of the natural history of metastatic renal cell carcinoma and this might produce synergistic antitumor activity [1]. In the presence of extra pancreatic recurrence, improved survival rates have been achieved (5-year survival rate of 88%) by a combination of pancreatic resection and the use of immunotherapy [1].
Pancreatic metastases from renal cell carcinoma, although rare, are well documented and may be the only site of metastasis. They may present many years after resection of the primary renal cell carcinoma and, thus, should be looked for during the follow-up or in patients with upper abdominal symptoms. When possible, complete surgical resection offers the best chance of cure. Local recurrence or a new site of tumor development in the pancreas may be treated actively with a total pancreatectomy. Previously resected renal cell carcinoma metastasis at other sites, should not discourage aggressive treatment of an isolated pancreatic secondary tumor. Surgical treatment of pancreatic metastasis from neoplasms other than renal cell carcinoma has a poor prognosis.
The authors have no potential conflicts of interest