- (2016) Volume 17, Issue 1
James Matthew Lloyd Williamson, Newton ACS Wong, Andrew D Stickland
Departments of Hepatopancreatobiliary Surgery and Histopathology, Bristol Royal Infirmary, Upper Maudlin Street, Bristol, BS2 8HW
Received July 25th, 2015 - Accepted September 30th, 2015
Introduction Pancreatic lymphoepithelial cysts are rare benign pancreatic lesions that mimic more common neoplastic mucinous tumours. Preoperative diagnosis may be uncertain, which leads to subsequent resection and confirmation of their identification on pathological examination. Endoscopic ultrasound and biopsy can indicate the nature of this pancreatic cyst, therefore precluding the need for operative intervention. Methods Four consecutive patients with a pancreatic lymphoepithelial cyst were identified over a two-year period (March 2012 to February 2014); three from resected specimens and one from endoscopic ultrasound guided fine needle aspiration biopsy. Details of patient demographics, investigations and management were retrospectively analysed. Results Four male patients aged 45-69 years (mean 59 years) were identified; three underwent pancreatic resection (two distal pancreatectomies and one median pancreatectomy) and one was diagnosed following endoscopic ultrasound guided biopsy. Pathological examination revealed keratin and squamous epithelium in all cases consistent with a lymphoepithelial cyst. Conclusions Lymphoepithelial pancreatic cysts are rare and present a diagnostic dilemma. Definitive preoperative diagnosis is seldom achieved due to their non-specific radiological and endoscopic ultrasound features, combined with variations in tumour marker expression and difficulties in achieving cytological and histological assessment. As a result these lesions tend to be over-treated with pancreatic resection. An increasing awareness of these cysts and the role of endoscopic ultrasound may allow conservative management.
Pancreatic Cyst; Pancreatectomy
Lymphoepithelial cysts are rare benign pancreatic lesions that account for 0.5% of all pancreatic cysts [1-2]. They have a male predominance and are typically detected in the fifth decade [3-4]. Most lesions are detected incidentally, although some patients have symptoms associated with their mass effect [3, 5]. Computed tomography and tumour markers are seldom indicative, and patients are frequently misdiagnosed as harbouring a potentially neoplastic lesion resulting in subsequent resection [6-7]. Endoscopic ultrasonography combined with histological or cytological assessment may be diagnostic if squamous or keratinous material is present [7-8]. In cases where diagnostic uncertainty persists, operative resection is advocated and this may include minimally invasive pancreatectomy. Typical histological features include squamous epithelial lining, with underlying prominent lymphoid tissue [4, 6]. Outcomes are excellent given the benign behaviour of these lesions. We report our experience of managing pancreatic lymphoepithelial cysts and the influence of endoscopic ultrasonography in providing a non-resectional diagnosis.
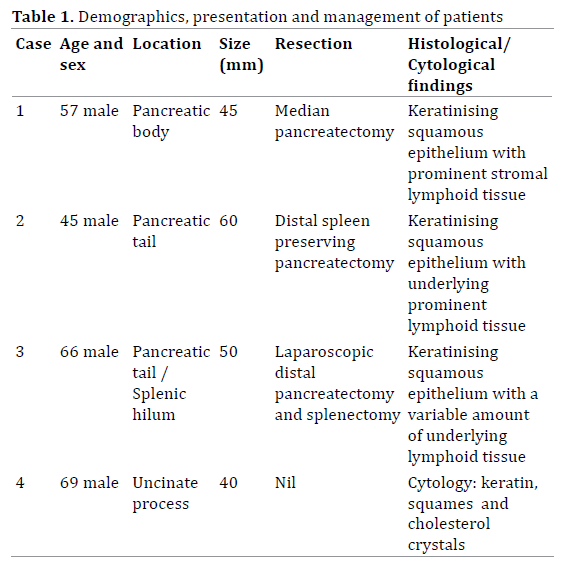
Four consecutive cases of pancreatic lymphoepithelial cysts were identified at our institution over a two-year period via the hepatopancreatobiliary multidisciplinary team meeting. Three were confirmed following pathological examination of resected specimens and one following endoscopic ultrasound (EUS) guided fine needle aspiration biopsy (with subsequent cytological and immunohistochemical analyses). All four patients were male, with an age range from 45-69 years (mean 59 years). The demographics, presentation and management of these patients were retrospectively analysed and discussed below (Table 1). Immunohistochemical analysis of the squamous epithelium (using polyclonal antibodies) detected varying expression of CEA in all specimens.
Case #1
A Fifty-seven-year old man had an incidental peripancreatic lesion detected during computed tomography (CT) scanning of his chest for underlying bronchiectasis. He was asymptomatic and had no other past medical history. Routine blood tests were unremarkable apart from a mildly elevated serum Ca19-9 of 36 KIU/L (normal = <35 KIU/L). Review of his CT scan confirmed a low-density, multiloculated lesion located between the stomach and pancreas. Urgent endoscopic ultrasound (EUS) revealed biliary sludge and confirmed that the cyst was arising from the region of the pancreatic body, but appeared to be separate to the pancreas itself. The EUS findings raised the possibility of a mucinous lesion, an inflammatory process or an organised necrotic collection. Fine needle aspiration of the cyst revealed an amylase of 72 units (normal = <100 IU/L) and an elevated CEA level of 5500ugL (normal = <5). Cytological assessment revealed a large amount of cellular debris and inflammatory cells, but no mucin was identified.
Given the uncertain aetiology of the lesion and the elevated CEA, which was highly suspicious of a mucinous lesion, the patient proceeded to resection. At operation the cystic lesion was identified relating to the body of the pancreas and a median resection was performed combined with a cholecystectomy. Histological examination of the lesion revealed a thin walled, multiloculated cyst measuring 45mm in maximal diameter. The cyst lining was composed of focally keratinising benign squamous epithelium (which expressed CEA on immunohistochemical staining), with the underlying stroma containing prominent lymphoid tissue. These histological findings were interpreted as a completely excised lymphoepithelial cyst. The patient made a routine recovery with no signs of disease recurrence four months postoperatively.
Case #2
A Forty-five-year-old man was investigated with an abdominal CT for non-specific upper abdominal pains and bloating. There was no history of weight loss or jaundice, abdominal examination was unremarkable and initial investigations, including tumour markers and urinary catecholamines, were normal. The CT revealed a 7×6 cm retroperitoneal soft tissue density mass, with a fluid component, adjacent to the pancreatic tail, consistent with a cystic tumour (Figure 1). Urgent EUS confirmed a partly cystic lesion adjacent to, but not arising from, the tail of the pancreas. FNA revealed blood stained pus and necrotic fluid, consistent with an inflammatory process; biochemical analysis revealed a normal amylase level and an elevated CEA of 300 μg/L (normal = <5 μg/L). Cytological assessment showed acellular debris only with no microbial growth on culture. Given the EUS and fluid aspirate findings the over-riding diagnosis was one of a mucinous cystic tumour and thus the patient proceeded to laparotomy.
At operation the semi-cystic lesion was identified abutting the pancreatic tail. A distal spleen preserving pancreatectomy was carried out and the patient made a routine recovery. Histological examination of the lesion revealed a 60 mm complex, thin-walled unilocular cyst arising from the tail of the pancreas. The cyst was lined by focally keratinising benign squamous epithelium, with underlying prominent lymphoid tissue, consistent with a completely excised pancreatic lymphoepithelial cyst. Patchy expression of CEA by the squamous cells was noted on immunohistochemistry.
A Sixty-six-year old man with exertional dyspnoea secondary to severe chronic obstructive pulmonary disease underwent a CT colonoscopy in order to investigate epigastric pain, reflux, altered bowel habit, flushing and dizziness. The CT did not reveal any colonic pathology, but noted a 5 cm homogenous lesion within the pancreatic tail, consistent with a neuroendocrine tumour (Figure 2). A second 2.5 cm lesion in close proximity to the splenic hilum was also seen, which was suspicious of IPMN. Further investigations, in the form of a gut hormone profile and gastroscopy were unremarkable, and subsequent EUS could not reveal any further information, as the lesion was not in close proximity to the stomach. Tumour markers (CEA and Ca 19.9) were both normal and Octreotide scanning did not show enhancement of the lesion or any distal sites.
Given the uncertainty of the lesion he underwent a laparoscopic assisted distal pancreatectomy and splenectomy. At operation the lesion appeared separate to the pancreas and contained caseous material, akin to a sebaceous cyst. The second lesion appeared consistent with a splenunculus, and this was confirmed on histological examination. Pathological assessment of the primary lesion revealed a 50mm thin walled cyst, containing several daughter cysts, that abutted the pancreas. One of the daughter cysts contained a cheesy yellow material, consistent with the intraoperative findings. Histology revealed that the cysts were lined with keratinising squamous epithelium (patchy CEA expression on immunohistochemistry) with a variable amount of underlying lymphoid tissue (Figure 3). The cysts were fully excised and consistent with lymphoepithelial cysts. The patient returned to theatre three days later for laparoscopic washout of an intraabdominal haematoma. He subsequently developed a respiratory tract infection, requiring ventilation and subsequent tracheostomy insertion (prior to removal); after a protracted recovery he was discharged with no evidence of pancreatic insufficiency.
Figure 3. (a.). Giemsa stained air-dried slide of turbid fluid aspirated from Case #4. Multiple square/rhomboid cholesterol crystals are present with surrounding keratinous debris. b-d) Histological images of the resected cyst of Case #2. The low-power image (b.). demonstrates a complex cyst whereas the higher powered image (c.). shows a cyst lining of squamous epithelium with underlying lymphoid tissue and deep to this, a focal foreign body reaction related to cholesterol clefts (examples are arrowed). (d.). The superficial layers of the lining squamous epithelium express CEA protein as has been demonstrated immunohistochemically.
Case #4
A Sixty-nine-year-old man was noted to have an incidental pancreatic lesion detected during a CT colonoscopy (for six weeks of rectal bleeding). He was otherwise asymptomatic and denied any abdominal pain, weight loss or abdominal distension. Examination was unremarkable and urgent pancreatic CT confirmed the presence of a 4cm low density lobulated cystic lesion projecting inferiorly from the pancreatic process. The lesion contained septation, with apparent central calcification. Serum tumour markers revealed an elevated ca 19.9 of 104 KIU/L (normal = <35). Given the uncertain aetiology of the lesion, urgent EUS was arranged. EUS revealed a well-defined multiloculated cystic lesion in the uncinate process, without obvious calcification. The lesion displaced, but did not communicate with, the pancreatic duct. Fine needle aspiration was performed, and this revealed a turbid fluid aspirate containing debris, which raised the possibility of a pseudocyst. Fluid microscopy was negative and, unfortunately, analysis of the fluid for CEA and Ca 19.9 was not possible due to the limited sample aspirated. Cytology showed keratin, squamous epithelium and cholesterol crystals (Figure 3) with subsequent immunohistochemistry confirming the presence of degenerate squamous epithelium within the cyst’s contents and some expression of CEA on immunohistochemistry. Differential diagnoses of dermoid cyst and metastatic or primary squamous cell carcinoma were considered unlikely in view of relative probability and the radiological findings of a cystic lesion. Therefore, these cytological findings were regarded to represent origin from a lymphoepithelial cyst. The patient remains well and under clinical surveillance and a further CT will be performed at 12 months to check that there is no radiological progression.
Pancreatic lymphoepithelial cysts are rare, benign lesions of uncertain aetiology and just over 100 cases have been reported in the literature since they were first described in 1985 [1-2, 9]. Lymphoepithelial cysts can be either unilocular or multilocular and can arise throughout the pancreas and often protrude outside it [7]. They are lined by mature, keratinizing squamous epithelium with surrounding lymphoid tissue [7]. Pancreatic lymphoepithelial cysts have a male predominance (with a ratio of 4:1) and are typically detected within the 5th decade (range 20-82 years) [3-4]. Lesions range in size from 0.5- 19 cm, with a mean diameter of 4.1 cm [3, 6]. The majority of cases are detected incidentally (65%), but associated symptoms include abdominal pain, a palpable mass, diarrhoea, anorexia, weight loss, nausea and vomiting [3, 5]. They have no clear associations, although systemic illness, including Sjogren’s syndrome, lymphoma and viral infections (particularly human immunodeficiency virus (HIV)) have been linked to lymphoepithelial cysts of the head and neck [10]; to date only one report exists in the literature associated with HIV and a pancreatic lesion [11]. It is thought that more pancreatic lymphoepithelial cysts are being detected in part due to the increased frequency and sensitivity of cross-sectional imaging combined with the increasing use of minimally invasive or partial pancreatic resections [6]. Most cases are diagnosed postoperatively, as preoperative diagnosis can be uncertain and they mimic mucinous cystic neoplasms [7].
The pathogenesis of pancreatic lymphoepithelial cysts is not fully understood, although three main hypotheses for their development exist. Firstly, the development of squamous metaplasia of an obstructed pancreatic duct that protrudes into a peripancreatic lymph node [12-13]. Secondly, that the cysts develop from ectopic pancreatic tissue within peripancreatic lymph nodes, which could explain the extrinsic location of the lesions and the fact that pancreatic tissue has been found throughout previous specimens [12-16]. Thirdly, that the lesions originate from misplaced brachial cleft cysts that have fused with pancreatic anlage during embryogenesis, which would account for the histological similarities between pancreatic lymphoepithelial cysts and branchial cleft cysts of the neck [2, 17].
Haematological investigation adds little to the overall diagnosis and tumour markers may be elevated with non-specific sensitivity [7, 18]. Ultrasound, computed tomography and magnetic resonance imaging can all be used to diagnose pancreatic lesions; although radiology cannot consistently separate lymphoepithelial cysts from neoplastic mucinous cysts (including mucinous cystic neoplasms and intraductal papillary mucinous neoplasms) [7]. Radiological findings that support a diagnosis include a well-defined, low attenuation mass, attached to the surface of the pancreas and not associated with pancreatic duct dilation [6]. Specific diagnosis features seen on MRI include hypointensity of the cystic wall and septations on T1 weighted images, with subsequent enhancement following contrast on T2 weighted images [6]. Keratinized material within the lymphoepithelial cyst can be seen and is associated with increased density on a precontrast CT scan, high signal intensity on T1 and low signal intensity on T2 weighted MRI images [19].
Endoscopic ultrasound (EUS) and EUS-guided biopsy (FNA cytology or core biopsy) coupled with both biochemical or tumour marker studies are being increasingly used to assist in accurate diagnosis of pancreatic cystic lesions [20-22]. EUS appearances vary from a solid to cystic mass, or mixed solid-cystic characteristics [8]. Despite the limitations of FNA assessing small numbers of cells that are typically present within a cyst (and thus being insensitive), they can be useful if certain features are found [7]. If squamous or keratinous material is present then FNA can support the diagnosis of lymphoepithelial cysts, but it can yield inconclusive results [7, 20]. Aspirates from lymphoepithelial cysts have been reported as being thick milky, cream or frothy and, as such, if these are found then they should raise the suspicion of the diagnosis [8, 10]. Analysis of cyst fluid analysis for both CEA and Ca 19-9 from lymphoepithelial cysts has been variable; high concentrations of CEA and Ca 19-9 have been reported [23]. A core biopsy of the cyst wall may show a mature keratinized squamous epithelium lining with underlying lymphoid tissue [24]. The presence of elevated CEA and Ca 19-9 is postulated to be due to squamous cells that express CEA (Figure 3) and Ca 19-9 [7]. In addition, previous studies have shown that the epithelial cells lining the cysts are immunoreactive to CEA and Ca 19-9 in a similar manner to those lining the pancreatic ducts [23, 25]. No definitive correlation between the degree of immunohistochmical expression of CEA by the squamous epithelium and the amount of CEA found within a cyst has been reported.
Given the lack of accurate reliable preoperative diagnosis, most lymphoepithelial cysts are diagnosed on pathological examination. Macroscopic assessment shows that lymphoepithelial cysts are well defined, rounded lesions with well-defined with clear demarcation margins from surrounding pancreatic or fatty tissue [8]. Lesions may contain cheesy, granular, brown-yellow material, as in our third case [10]. They have an equal distribution between multi- and unilocular cysts and are equally distributed within the pancreas [12-13]. Histological examination confirms their uni- or multilocularity and reveals that the cysts are lined with keratinized squamous epithelium surrounded by a fibrous wall that contains lymphoid tissue [6]. These lesions have been associated with sebaceous cysts. Operative resection is curative given the uniform benignity of the lymphoepithelial cysts. The type of pancreatic resection is dependent upon its location and pancreatic sparing or minimally invasive techniques should be considered [26]. To date, no reports of cyst recurrence have been reported in the literature.
The authors have no conflict of interest to declare.