- (2015) Volume 16, Issue 4
Abhinav Agrawal , Sayee Sundar Alagusundarmoorthy and Sarfaraz Jasdanwala*
Department of Medicine, Monmouth Medical Center, Long Branch, NJ. USA
Received: March 27th, 2015 Accepted: April 28th, 2015
Elevation of pancreatic enzymes is often observed in patients admitted to intensive care units in the United States. Elevated pancreatic enzymes can occur due to acute pancreatitis or numerous non-specific reasons. Non-specific enzyme elevation can be seen in patients with head injury, acute renal failure, diabetic ketoacidosis or patients on hemodialysis. Patients with severe acute pancreatitis can be admitted to the intensive care units for intensive care or patients admitted to the intensive care units for other critical illness can develop acute pancreatitis due to a variety of reasons like ischemia, hypoperfusion, drugs or hypercalcemia. It can be a challenging task to distinguish between acute pancreatitis and non-specific enzyme elevation, especially in critically ill patients with multiple co-morbidities admitted to the intensive care units in whom historical information may not be always available. In addition, the clinical consequences of pancreatic enzyme elevation in the critically ill patients are also not very clear. This review attempts to describe the complex interplay of various factors that can lead to either pancreatic inflammation and/or pancreatic enzyme elevation in the critically ill patients along with the clinical consequences and approach to patient with pancreatic enzyme elevation in the intensive care units.
Amylases; Critical Care; Intensive Care Units; Lipase; Pancreatitis
Elevation of pancreatic enzymes occurs in 14-80% of the 5 million patients admitted to Intensive Care Units (ICU) in the United States [1-3]. Elevated pancreatic enzymes can occur due to Acute Pancreatitis (AP) or numerous non-specific reasons. Non-specific enzyme elevation can be seen in patients with head injury, acute renal failure or patients on hemodialysis [2]. AP, on the other hand, is also not uncommon in ICU patients. Patients with severe AP can be admitted to the ICU for intensive care or patients admitted to the ICU for other critical illness can develop AP due to a variety of reasons discussed in subsequent sections of this article.
The evaluation of elevated pancreatic enzymes in the critically ill patients can therefore be difficult as it becomes challenging to discern true pancreatic inflammation from non-specific enzyme elevation. This is compounded by the fact that a significant number of patients in the ICU are sedated, intubated or comatose. It is often not possible to elicit history regarding abdominal pain in such patients. Unless elevated levels of amylase and lipase are seen on routine blood work, pancreatic involvement in such patients may go undiagnosed for several days. On the other hand, non-specific elevations of serum amylase and lipase may result in an erroneous diagnosis of pancreatitis in such patients and subject the patient to further workup and treatment, which has potential risks involved. In addition, the clinical consequences of pancreatic enzyme elevation in the critically ill patients are also not very clear.
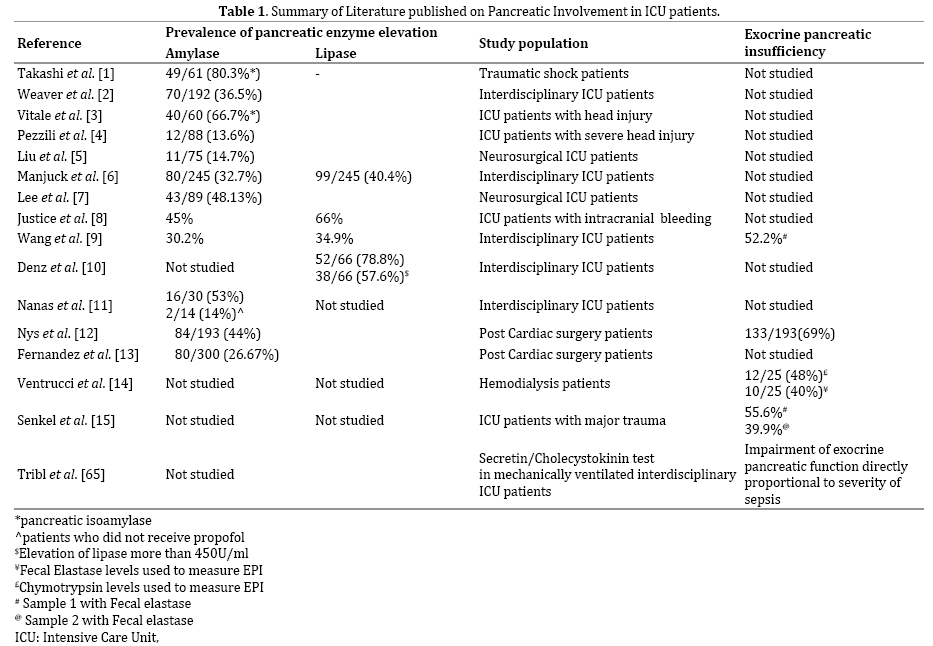
This review attempts to describe the complex interplay of various factors that can lead to either pancreatic inflammation and/or pancreatic enzyme elevation in the critically ill patients along with the clinical consequences and approach to patient with pancreatic enzyme elevation in the ICU. Table 1 lists the studies in literature regarding pancreatic involvement in ICU patients.
AP In Critically ill Patients
Patients who are admitted to the ICU for critical care management can develop AP. Described below are the several mechanisms by which such critically ill patient can develop pancreatic inflammation in the ICU.
Ischemia, Hypoperfusion and Sepsis
Pancreas has been postulated to suffer ischemic injury during periods of hypotension and tissue hypoperfusion. While ischemia can worsen AP from mild to severe, it can also primarily induce AP [4]. Thirty years ago, Popper et al. demonstrated that pancreatic duct ligation produces not only edema, but also that clamping of the pancreatic arterial supply, normally a harmless maneuver, converts pancreatic edema into pancreatic necrosis [5]. Warshaw et al. [6] demonstrated an extraordinary incidence of major pancreatic injury occurring in patients who have been in shock. They showed a positive correlation of AP with the presence of renal ischemic injury (ATN), also a manifestation of hypoperfusion. Multiple mechanisms have been proposed to cause pancreatic inflammation due to ischemia.
Pancreatic inflammation can occur due to arterial narrowing or occlusion as seen in polyarteritis nodosa [7].
This can lead to ischemia-reperfusion leading to generation of free radicals inducing pancreatic injury [8]. Ischemia can also lead to altered microcirculatory perfusion [9]. This can result in ectopic trypsinogen activation due increased intracellular and intramitochondrial calcium concentration causing lethal AP [10, 11]. Apoptosis has also been proposed as a possible mechanism of AP in patients with sepsis, though limited evidence is available regarding the same.
The hypoperfusion to the pancreas is magnified by the fact that blood is preferentially redistributed away from the pancreas in sepsis [12]. Pancreatic inflammation, which starts with ischemia to the pancreas, tends to lead to severe and often irreversible multi-organ damage including cardiovascular collapse due to secretion of vasoactive substances. Furthermore development of tissue necrosis due to ischemia can trigger disseminated intravascular coagulation and provide medium for secondary bacterial infection leading to fatal systemic complications including death [6] Conventional fluid therapy and maintaining the mean arterial pressures by means of optimizing blood pressure can provide effective means of recovery in such patients [13].
Drugs
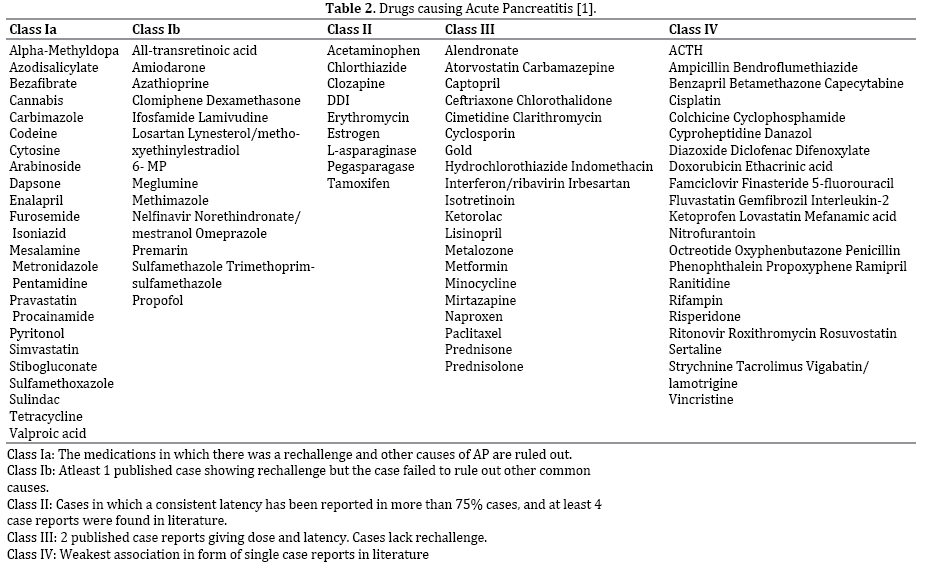
Drugs are responsible for 0.1%-2% of the total incidence of AP. The cause of drug induced pancreatic injury is unknown. These drugs can be divided into those with intrinsic toxicity of the pancreas or those than cause damage by the means of an idiosyncratic reaction [14]. Many drugs used in the ICU have the potential to cause pancreatic injury and AP. Propofol, a lipid based sedative hypnotic agent, has been implicated as the cause of AP in several case reports [15-17]. Propofol is used for induction of general anesthesia and for long term sedation in mechanically ventilated patients. In a study by Nanas et al. [18], 16 (53%) out of the 30 patients on propofol were found to have a significant increase in amylase. Of the other 14 patients who did not receive propofol, only two (14%) patents developed hyperamylasemia (P=0.021). Hypertriglyceridemia from propofol has been described as a putative mechanism, though not all cases of propofol associated pancreatic inflammation can be explained by it, especially those occurring after a single bolus used for induction of anesthesia [17]. Muniraj & Aslanian have suggested that propofol might cause AP by the way of an idiosyncratic drug reaction in patients without elevated triglyceride levels [19]. Thus serum triglyceride concentrations should be routinely monitored in patients on propofol and it is critical to consider alternative sedation strategies if hypertriglyceridemia is detected [20]. The use of vasoconstrictors for patients presenting with shock and hypotension has also been reported to cause pancreatic damage due to splanchnic ischemia [21]. Another commonly used drug in the ICU are steroids. The use of steroids like dexamethasone, prednisone and cortisone has also been implicated in causing AP in a series of case reports [14, 22]. While more than half of these patients died of AP thus indicating a severe disease, these case reports failed to clearly exclude other potential confounding factors [14]. The complete list of drugs causing acute pancreatitis has been mentioned in Table 2.
Hypercalcemia
Hypercalcemia is frequently encountered in patients in the ICU, especially in patients with renal failure and burns [23, 24]. Hypercalcemia can occur in critically ill patients due to dehydration, immobilization, supplementations and diuretics. Bai et al. reviewed ten retrospective studies, each with >50 patients and found that the rate of pancreatitis was higher in eight studies in patients with higher serum calcium levels [25]. Hypercalcemia leads to increased cytosolic calcium concentrations in the pancreatic acinar cells, leading to premature trypsinogen activation leading to pancreatic inflammation and thus causing AP [25, 26].
Cardiac and Abdominal Surgeries
Cardiac surgery leads to an inflammatory state leading to neutrophil activation and lymphocyte functional dysregulation that plays a key role in the pathogenesis of AP in patients undergoing cardiac surgery [27-29]. Pancreatic enzyme elevation after cardiac surgery occurs in a biphasic fashion as shown by Monique et al. [30]. The first peak is attributed to ischemia or a perioperative inflammation reaction, and the second to inflammation. A perioperative inflammation reaction with cellular acidosis and stimulation of neutrophils is proposed to be due to the surgical procedure and to the contact of blood with the material of the cardio-pulmonary-bypass circuit, increasing the adherence of phagocytes to the vessel walls. Splanchnic hypoperfusion could also originate from the mechanical compression of the splanchnic area during the surgery causing pancreatic injury. Similarly, AP can develop after major abdominal surgeries and in patients with a ruptured abdominal aortic aneurysm. In a series of 25 patients with ruptured aortic aneurysm, hyperamylasemia was associated with 10 patients and was associated with significantly poor outcomes (P=0.005) [31]. AP in such patients can occur due to perioperative manipulation of pancreas, release of inflammatory mediators due to extensive tissue injury or subsequent bacterial injury [31-35].
Conflicting evidence exists about the role of MRI contrast agents a putative etiology for AP. Blasco-Perrin et al. recently demonstrated that re-challenge with a MRI contrast agent adobenate imeglumine can precipitate AP [36-39]. Several other reports have shown Gadolinium to induce AP or worsen pre-existing AP. On the other hand, Schneider et al. [40] have shown based on animal model experiments that Gadolinium chloride reduces both microcirculatory and morphological pancreatic damage in alcohol induced pancreatitis. These results could not be replicated in a follow up animal study by Dda et al., which failed to show any benefits of use of gadolinium chloride in subjects with acute pancreatitis [41]. Thus, the role of contrast in causing pancreatic injury remains controversial
Patients with severe AP requiring ICU care
Patients with severe AP need aggressive monitoring and supportive care in the ICU. Clinical examination and signs of organ failure can assess the severity of AP. Objective assessment of the severity can be performed by the measurement of APACHE II score and systemic inflammatory response syndrome (SIRS) score [42]. Initial management of a patient with AP consists of supportive care with fluid resuscitation. This includes aggressive hydration and frequent reassessment of fluid requirements. The preferred replacement fluid in such patients is Lactated Ringer’s solution. Enteral feeding rather than parenteral nutrition is recommended in patients with moderately severe and severe AP. In patients with gall stone pancreatitis, urgent ERCP is recommended followed by cholecystectomy in all operable patients. ERCP should be performed within 24 hours of admission in patients with AP and concurrent acute cholangitis. Routine use of antibiotics is not recommended in patients with severe AP or sterile pancreatic necrosis. Antibiotic therapy can be initiated in patients with necrosis who show clinical deterioration or in patients with suspected infected necrosis. The mortality rate ranges from 3 percent in patients with interstitial edematous pancreatitis to 17 percent in patients who develop pancreatic necrosis [43, 44]. In cases of stable infected necrosis, drainage should be delayed for more than four weeks to allow for development of walled-off necrosis. Immediate necrosectomy is performed if the patient remains symptomatic with infected necrosis [1].
Head Trauma, Intracranial Bleed and Neurosurgical Patients
Elevated levels of amylase and lipase have been observed in head trauma patients and patients with intracranial bleed. In a retrospective study of 75 patients from a neurosurgical ICU, elevated pancreatic enzymes were observed in 15% of the patients [45]. A slightly higher incidence was seen in patients with craniotomy (24%) and in patients with intracranial bleed (29%). In a study with 74 patients with severe head injury with a Glasgow coma scale less than 10, total amylase was elevated in 38% of the patients and pancreatic iso-amylase was elevated in 61% of the patients [46]. Finding of elevated pancreatic enzymes in these patients has led to the theory of centrally activated pathways causing enzyme elevation. Cephalic activation of pancreatic exocrine secretion is vagally mediated. This vagal activation may be suppressed by vasoactive intestinal polypeptide neutralization as well as antagonists to cholinergic receptor, cholecystokinin A receptor or gastrin releasing peptide receptor [47]. Thus both post ganglionic and peptidergic pathways through CNC activation appear to be important in the stimulation of exocrine pancreatic enzyme secretion. The central activation mechanism may be responsible for elevated exocrine pancreatic secretion and possible inflammation due to head trauma or other intracranial events [45]. Also, sympathetic control of pancreatic secretion is primarily inhibitory via vasoconstriction, which limits the maximal rate of secretion. Loss of sympathetic tone with resultant hyperperfusion may result in increased amylase production after head trauma [48]. Lee et al. also noted a 48% incidence of elevated pancreatic enzymes in 89 patients admitted to the neurosurgical intensive care unit with gastrointestinal symptoms. Interestingly, elevated pancreatic enzymes were associated with higher mortality in these patients. Since there was no evidence of direct pancreatic damage or abdominal trauma in these patients, the authors concluded the hyperenzymemia was probably due to centrally activated pathways [45].
Diabetic Ketoacidosis (DKA)
Non-specific elevation of pancreatic enzymes has been noted in patients with DKA. Yadav et al. studied 150 consecutive episodes of DKA in 135 patients and noted non-specific elevation of amylase in 16 % patients and nonspecific elevation of lipase in 24% patients [49]. Haddad et al. compared pancreatic enzyme elevations in children with and without and observed the hyperlipasemia was more common in the DKA arm [50]. Nair et al. also studied 100 consecutive episodes of DKA and performed CT scan of the abdomen for all patients with abdominal pain or elevated pancreatic enzymes or elevated triglyceride levels>500 mg/dL. They found elevated lipase in 29% cases and elevated amylase in 21% cases. Only 11% of these cases had pancreatic involvement evident on abdominal CT scan [51]. Factors that correlate with elevated levels of amylase and lipase include hyperglycemia, dehydration and acidosis [52]. Acidosis can lead to enhanced calcium signaling which, in turn, and can lead to pancreatic damage in patients with DKA [53, 54]. While the exact pathogenesis remains unclear, profound hyperlipidemia is also an identifiable factor [51]. It is also important to remember that the findings in DKA i.e. abdominal pain and pancreatic enzyme elevation may be masking a co-existing AP.
Chronic Renal Failure (CRF)/Hemo-dialysis (HD)
The exact mechanisms of serum amylase and lipase metabolism are still not fully understood. The kidneys are assumed to play a major role in their metabolism. The glomerular filtration rate of both these enzymes is nearly the same. Lipase is subsequently completely reabsorbed in the tubules as opposed to amylase [55]. Elevation of amylase and lipase in patients with renal failure can be due to impaired renal clearance [56]. Elevated levels of lipase are seen in HD patients without evidence of true AP [57]. In contrast, dialysis does not seem to alter serum amylase levels. The cause of increased serum lipase in dialysis patients may be related to the use of heparin during dialysis. This post dialysis heparin induced elevation of serum lipase can be attributed to the heparin induced increased lipolytic activity, heparin’s increased cross reactivity with lipoprotein lipase or its ability to induce endothelial lipoprotein lipase. Consistent with this explanation is the fact that when heparin is replaced with alternate anticoagulants like nafamostat mesylate and defibrotide, post dialysis rise in serum lipase is not observed [57, 58]. Hyperamylasemia has also been noted in patients with acute liver failure. The enzyme elevation in such patients has been attributed the associated renal dysfunction and multi-organ failure [59].
Chosidow et al. first reported an increased incidence of hyperamylasemia in patients with TEN. In their study, most of the patients had increase in predominantly salivary isoamylase and normal lipase levels [60]. This indicates that the source of elevated amylase may be the salivary glands rather than the pancreas. Subsequently, in a retrospective review of 24 pediatric patients admitted with TEN/SJS, 4 patients were noted to have elevated amylase and lipase levels without any evidence of clinical pancreatitis. Isoamylase levels were not checked in these patients though a concurrent rise in amylase and lipase levels may indicate a pancreatic source. These patients with elevated pancreatic enzymes also had a higher incidence of sepsis, which might be the source of pancreatic injury [61]. Thus, while elevated amylase and lipase levels might be seen in patients with TEN and SJS, the source of such rise in the enzymes remains controversial.
Exocrine Pancreatic Insufficiency (EPI) in the ICU
EPI in patients admitted to the ICU has been studied extensively with the reported incidence between 48- 55.6% [62-64]. Fecal elastase levels have been used to assess the presence and degree of EPI. It was seen that EPI is not associated with elevation of pancreatic enzymes or presence of any morphological damage to the pancreas detected by radiological investigations. Senkal et al. [63] reported severe EPI in critically ill patients after severe trauma. 3 out of the 18 patients in their study had severe EPI (fecal elastase less than 100 μg/g stool) whereas 7 out of the18 patients had moderate EPI (fecal elastase levels less than 200 μg/g stool). Ventrucci et al. [64] demonstrated a high prevalence of EPI in patients requiring hemo-dialysis. Tribl et al. [65] performed secretin – cholecystokinin tests in 21 critically ill, mechanically ventilated patients and found significantly reduced content of amylase and chymotrypsin in duodenal juice compared with healthy controls. They also reported a significant correlation between amylase secretion, trypsin secretion and APACHE III, SOFA scores indicating that degree of pancreatic cellular dysfunction was proportional to the degree of sepsis. The presence of associated EPI indicates the presence of pancreatic dysfunction and cellular damage in these patients.
A number of tests are available to assess exocrine pancreatic insufficiency in critically ill patients. The tests can be broadly classified into direct invasive tests to measure pancreatic enzymes and indirect tests to assess the function of pancreas. These tests along with their relative advantages and disadvantages are summarized in Table 3. Direct tests involve the aspiration of pancreatic contents with secretin or cholecystokinin/cerulein administration and thereby assess the function. Due to the invasiveness of these tests, it’s available only in few centers and is not indicated in regular clinical practice [66]. Indirect tests include measurements of fecal elastase 1 [67, 68], N benzoyl L tyrosyl p aminobenzoic acid and Pacreolauryl test [69, 70], Sudan Staining – [71], Steatocrit [72, 73], Van De Kramer method [74], Coefficient of fat absorption (CFA) [75] and Nutritional markers [76]. A number of tests such as D–Xylose test for carbohydrate metabolism, C– Triolein breath test and fecal chemotrypsin excretion have also been proposed to assess the various components of exocrine pancreatic insufficiency. To assess the nutritional status and long term follow up in these patients measuring the bioelectrical impedance phase angle [77] has also been proposed in these patients. The study was largely done in patients with pancreatic cancer and their assessed nutritional status was directly proportional to the degree of pancreatic function.

EPI can be commonly encountered in critically ill patients. It has also been noted that malnutrition occurs in 38-88% of ICU patients. Little attention has been paid to the potential contribution of EPI to malnutrition. Further studies needs to be designed to investigate effects of pancreatic enzyme supplementation on malnutrition in critically ill patients [78].
Clinical Consequences of Pancreatic Enzyme Elevation in Critically ill Patients
Limited numbers of studies in the past have analyzed clinical outcomes in critically ill patients who have elevated pancreatic enzyme levels. In a retrospective study by Manjuck et al. [79], elevated lipase and amylase levels were associated, not necessarily causally, to an increased length of stay and duration of mechanical ventilation (MV). Outcomes were compared between two groups – One with normal lipase levels and another with lipase elevation just above upper normal limit (ULN). As per the current practice guidelines from the ACG [1] lipase level >3 times ULN is considered diagnostic of AP. Hence, it is unclear how many patient in the elevated lipase group actually had true pancreatic inflammation. In some of the patients in the elevated lipase group, non-specific causes of lipase elevation other than AP such as renal insufficiency, DKA, liver disease might have been the reason for enzyme elevation. Regardless of the etiology of lipase elevation, this group was found to have increase in length of hospital stay and increase in the duration of MV. However, the difference in mortality between the 2 groups was not significant. Liu et al. also reported longer hospital and ICU stay in patients with hyperlipasemia [45].
In another study by lee et al. 89 patients admitted to a neurosurgical ICU were studied [48]. They found that patients with higher lipase and amylase levels had a significantly high mortality rate. However, the elevation of pancreatic enzymes was not associated with a longer ICU stay (P=0.538) or the duration of MV (P=0.7). This study has some limitations. The sample size was relatively small. The study only involved neurosurgical patients and thus the results cannot be extrapolated to all ICU patients.
In both the studies discussed above, elevated enzyme levels cannot be definitively attributed to AP because of the following reasons.
1. The upper normal limit for lipase levels was used as a cut of for the study instead of 3 times the upper normal limit, which is the current acceptable diagnostic cut off. Therefore, the real proportion of non-specific lipase elevations cannot be determined in these patients.
2. A higher proportion of renal failure (serum creatinine >1.5 mg/dL) was observed in patients with elevated lipase levels in the study by Lee et al..
3. The mortality in patients studied by Lee et al. was similar in patients with both positive and negative CT scan findings for AP (44.4% vs. 40%, P=0.094)
Due to these reasons, it cannot be concluded that mortality and morbidity in the elevated lipase group noticed in both the studies was resulting from true AP. It is possible that non-specific elevated lipase level in critically ill patients could be a marker of systemic inflammation rather than a disease process limited to the pancreas. This hypothesis could be tested by means of a prospective study with a larger cohort and inclusive of patients with wider pathology.
Summary and Approach to Patient
It can be a challenging task to distinguish between AP and non-specific enzyme elevation, especially in critically ill patients admitted to the ICU in whom historical information may not be always available and in whom, at the same time, multiple complex co-morbidities may exist to cause a non specific pancreatic enzyme elevation.
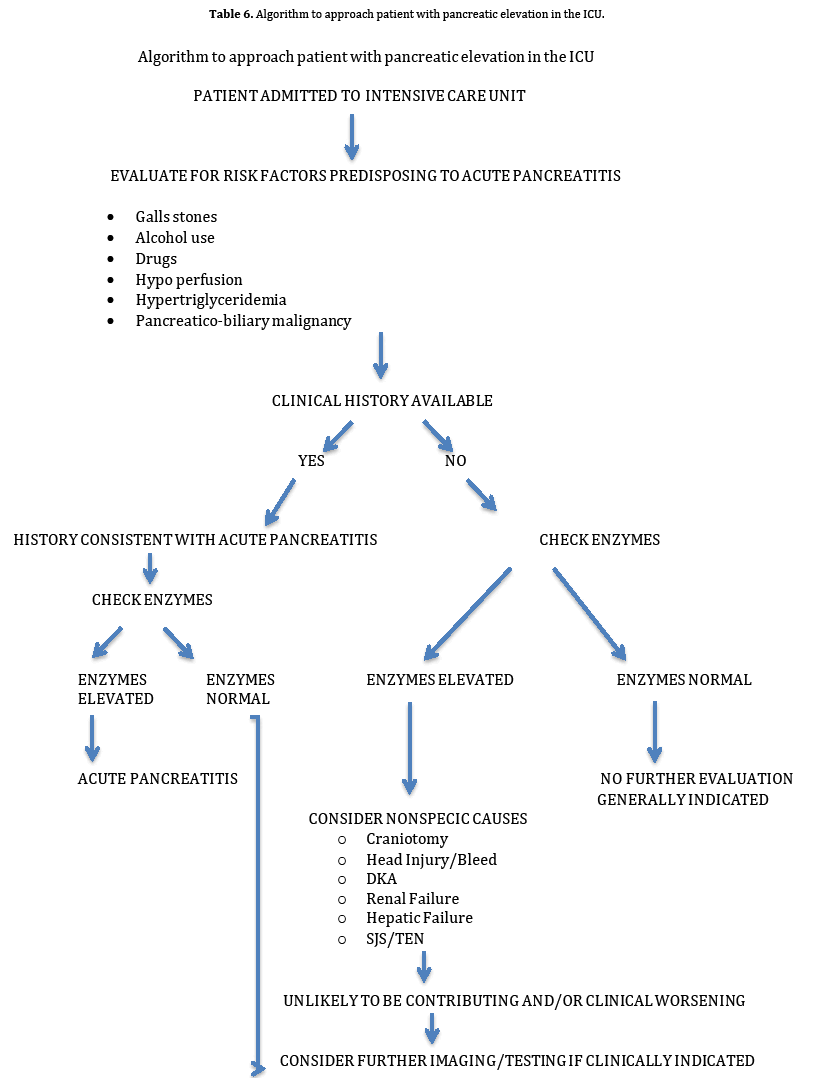
All patients admitted to the ICU should be evaluated for risk factors that can predispose them to development of AP. These risk factors are summarized in Table 4. In patients who have any of these risk factors, and in whom history of abdominal pain cannot be reliably elicited, it may be prudent to check pancreatic enzyme levels. If found to be elevated, causes for non-specific enzyme elevation, summarized in Table 5, should be looked into before further considering assessment or invasive treatment for AP. Algorithm describing an approach to a patient with pancreatic enzyme elevation in the ICU is summarized in Table 6.


Patients with severe acute AP who require monitoring in the ICU due to severity of AP require the same supportive care and as patients with mild to moderate AP. Additional special aspects of management of such severe cases is summarized in Table 7.

The clinical outcomes in ICU patients with elevated pancreatic enzyme levels regardless of etiology, although studied limitedly in the past, were found to be significantly different as compared to patients with normal or minimally elevated levels in terms of morbidity and length of ICU stay. Correlation between pancreatic enzyme elevation and mortality cannot be reliably determined due to paucity of data. Whether association of pancreatic enzyme elevation and higher morbidity and length of ICU stay is a result of true AP or whether pancreatic enzyme elevation is just a marker of systemic inflammation which leads to higher morbidity and length of stay, is a question that can be addressed by future prospective studies.
The authors had no conflicts of interest