- (2007) Volume 8, Issue 3
Thomas Becker, Bastian Ringe, Miguel Nyibata, Andreas Meyer zu Vilsendorf, Harald Schrem, Rainer Lück, Michael Neipp, Jürgen Klempnauer, Hueseyin Bektas
Department of General, Visceral, and Transplant Surgery, Medical School of Hannover Hannover, Germany
Received February 22nd, 2007 - Accepted March 16th, 2007
Context In clinical pancreas transplantation the choice of preservation solution may have an impact on graft pancreatitis. Experience with histidine-tryptophan-ketoglutarate (HTK) is still limited whereas University of Wisconsin (UW) solution is currently the preferred perfusate worldwide. Objective The aim of this study was to analyze our experience with HTK in pancreas transplantation. Participants In a retrospective analysis, data from 95 primary simultaneous pancreaskidney transplantations were reviewed. The use of HTK (n=48) and UW (n=47) solution was stratified into two groups. Main outcome measures Patient/graft survival and early graft function were compared. Results No significant differences between 1, 3 and 12 month patient survival (HTK: 97.9%, 97.9%, and 95.7% vs. UW: 95.7%, 89.4%, and 89.4%, respectively), and pancreas graft survival (HTK: 87.5%, 87.5%, and 85.4% vs. UW: 87.0%, 82.6%, and 82.6%, respectively) were detected. Higher values for peak lipase were observed on day 1 in the HTK group (not reaching significance: P=0.131) whereas no differences were noted for amylase and C-reactive protein. Conclusions HTK is clinically comparable to UW. Both solutions have been shown to be safe for pancreas preservation. Successful pancreas transplantation depends on many factors such as donor and recipient factors, but skilled organ procurement techniques, organ preservation, and transplant experience in this field is mandatory. The choice of organ preservation solution is only one point in this context.
Bretschneider cardioplegic solution; Pancreas Transplantation; University of Wisconsin-lactobionate solution
ATG: anti lymphocyte antigen/anti T-cell globulin induction therapy; HTK: histidine-tryptophan-ketoglutarate; UW: University of Wisconsin
The key to successful pancreas transplantation is a safe organ procurement and preservation technique. Graft thrombosis and severe graft pancreatitis are dreaded post-transplantation complications. University of Wisconsin (UW) solution is most commonly used as an abdominal perfusate worldwide; increasing use of histidine-tryptophan-ketoglutarate (HTK) has been shown to have similar outcomes after liver and kidney transplantation. Clinical experience in pancreas transplantation with HTK is limited [1, 2, 3, 4] and it is still controversial as to whether HTK is the appropriate perfusion solution in pancreas transplantation. The aim of this study was to analyze and to review our experience with HTK solution in pancreas preservation and transplantation.
Ninety-five patients who underwent primary simultaneous pancreas-kidney transplantation with portal venous drainage of pancreas allografts over a period of 6 years (from January 2000 to April 2006) were included in this study. Data from this cohort was stratified into two groups and organs perfused using HTK (n=48) and UW (n=47) were compared; early pancreas graft function as well as patient and graft survival were assessed. To determine early graft function and to verify clinical signs of graft pancreatitis, a daily diagnostic work-up including fasting glucose, amylase, lipase and C-reactive protein were performed on postoperative days 1-7.
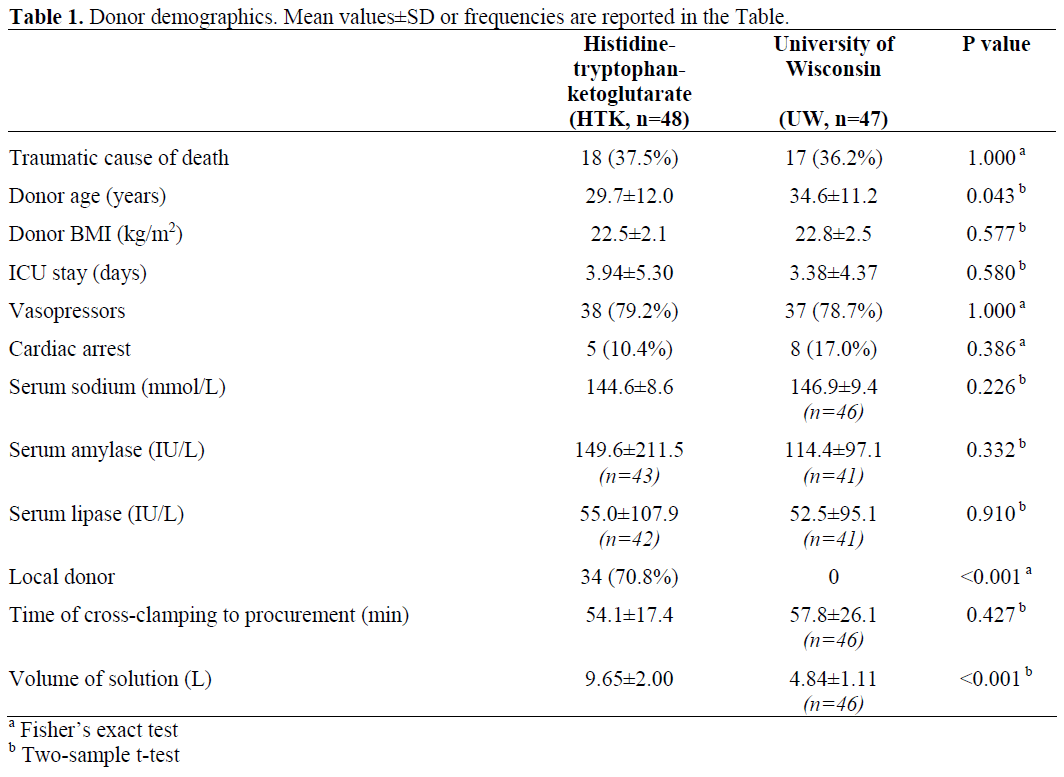
Donor Demographics
Donor demographics (Table 1) showed no significant differences regarding cause of donor death, BMI, ICU stay, vasopressors, cardiac arrest, as well as serum sodium, amylases and lipase while donor age differs significantly between the two groups (P=0.043). There was an expected difference in terms of local or external organ procurement: 34 out of 48 (70.8%) of the donors in the HTK group were procured locally and 14 (29.1%) were procured from other centers while, in the UW group, all pancreas procurements were performed by external teams (no usage of UW solution in Hannover). Furthermore, perfusion data showed no difference in time from crossclamping to pancreas procurement. However, the perfusion volume was expectedly higher, almost twice as high in the HTK group as compared to the UW group.
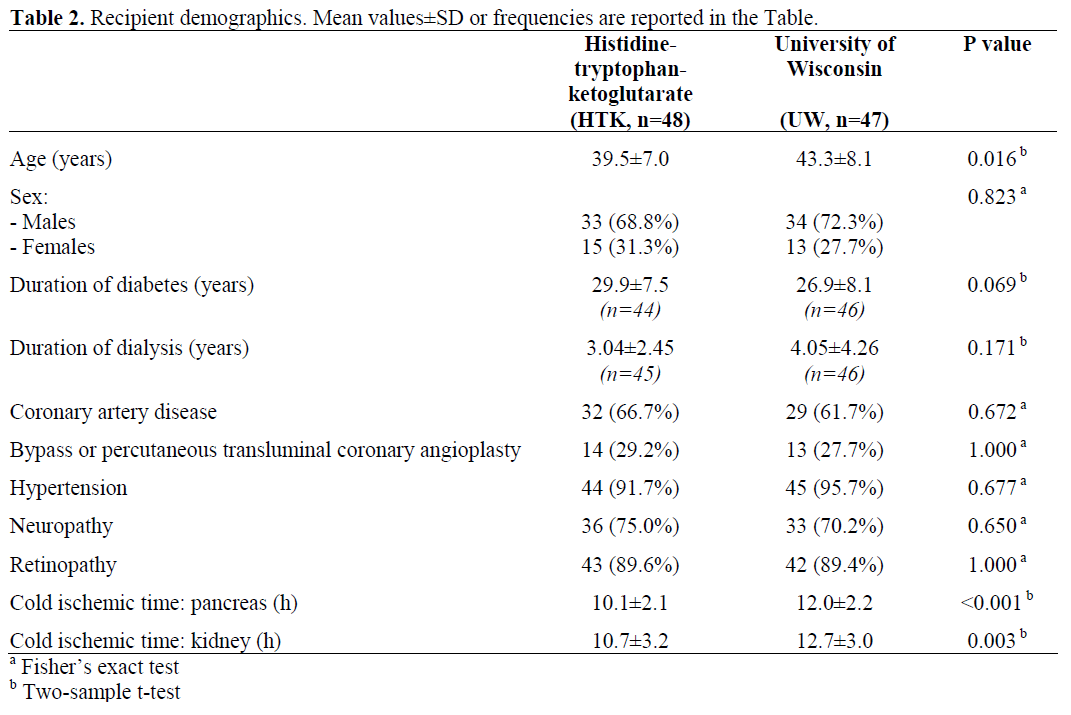
Recipient Demographics
Recipient demographics (Table 2) showed no significant differences in terms of gender, time of diabetes onset or duration of hemodialysis, coronary artery disease, bypass or percutaneous transluminal coronary angioplasty, hypertension, neuropathy and retinopathy. Age was significantly lower in the HTK group as compared to the UW group (P=0.016). UW-preserved pancreas grafts were exclusively procured externally with a slightly longer cold ischemic time of about two hours of both pancreas (HTK: 10.1±2.1 h vs. UW: 12.0±2.2 h;P<0.001) and kidney (HTK: 10.7±3.2 h vs. UW: 12.7±3.0 h; P=0.003) cold ischemic times.
Immunosuppression
All patients received anti-lymphocyte antigen/anti T-cell globulin (ATG) induction therapy (n=31) (thymoglobulin 2-5 mg/kg; Genzyme, Neu-Isenburg, Germany) or IL-2 antibody (n=64) (basiliximab 20 mg day 0 and 4; Novartis, Basel, Switzerland) followed by triple baseline immunosuppression with calcineurin inhibitor (CNI), mycophenolate mofetil (MMF) and steroids. Tacrolimus (n=50) (Prograf®, Astellas, Munich, Germany) doses were adjusted to achieve trough levels of 10-15 ng/mL in the first month and were reduced to 5-10 ng/mL in the following 5 months according to the clinical course. Cyclosporine-ME (n=45) (Optoral®, Novartis, Basel, Switzerland) doses were adjusted to achieve trough levels of 180-250 ng/mL and/or C2-level 800-1,200 ng/mL in the first month and were reduced to 120-180 ng/mL in the first six months. All patients received MMF (CellCept®, F Hoffmann La Roche, Basel, Switzerland) 2-3 g daily with subsequent dosage adjustments based on tolerability and side effects. Standard centre steroid protocol was used in all patients tapered down to 5 mg at month six.
This study was conducted in full conformance with the principles of the “Declaration of Helsinki” and with the laws and regulations of our country. Written informed consent was obtained from each patient in this study. Approval of the local ethics committee was obtained.
Data are reported as means and standard deviations (SD) or frequencies. Statistical analysis was performed using the two-sample t-test and the Fisher’s exact test, as appropriate. Patient and graft survivals were calculated using the Kaplan-Meier method, and the log-rank test was applied to compare survival between the two groups. Two-tailed P values less than 0.05 were considered statistically significant. Windows Access® 2004 was used as a database platform; Windows Office Excel® 2004 was used to gather all the data, and SPSS® 13.0 was used for statistical analysis.
Patient and Graft Survival
Patient survival rates at 1 month, 3 months, and 1 year were 97.9%, 97.9%, and 95.7%, respectively in the HTK group and 95.7%, 89.4%, and 89.4%, respectively in the UW group with no significant differences (P=0.219, log-rank test) (Figure 1). Pancreas graft survival rates at 1 month, 3 months, and 1 year were 87.5%, 87.5%, and 85.4%, respectively in the HTK group vs. 87.0%, 82.6%, and 82.6%, respectively in the UW group, also showing no significant differences between the groups (P=0.695, log-rank test) (Figure 2a). Kidney graft survival was also not significantly different between the two groups (97.8% in the HTK group and 95.7% in the UW group at each of the three time intervals; P=0.538, log-rank test; Figure 2b).
Two deaths occurred in the HTK group within the first three months: one was related to a myocardial infarction during the second postoperative month and the second patient died of unknown causes. Both patients died with functioning grafts. Five deaths occurred in the UW group: 3 from sepsis, 1 myocardial infarction 6 weeks postoperatively and related to a cerebrovascular accident one week after transplantation; again, all with functioning grafts.
In all, 15 pancreas grafts (15.8%) were lost between day 1 and day 90 after transplantation, 7 in the HTK group (14.6%) and 8 (17.0%) in the UW group (P=0.785). Causes of graft loss were as follows: in the HTK group, we observed graft pancreatitis (n=1), thrombosis (n=2) and rejection (n=4), and, in the UW group, graft pancreatitis (n=4), thrombosis (n=2) and rejection (n=2).
Graft Function
Initial graft function was observed in 92 patients (96.8%) and delayed graft function in 3 patients (3.2%), one in the HTK group and two in the UW group. At the time of discharge, all patients were insulinindependent.
Post-transplant laboratory analysis was performed on a daily basis, and markers for graft function showed comparable graphs for serum amylase and lipase, decreasing from day 1 to day 7. No significant difference (P=0.665) between the two groups was found in the peak serum amylase activity observed on postoperative day 1 (HTK: 548±507 IU/L vs. UW 589±419 IU/L; Figure 3) while peak serum lipase activity on postoperative day 1, although higher in the HTK group than in the UW group (2,187±3,244 IU/L vs. 1,296±2,295 IU/L; P=0.131; Figure 4), did not reach significance. In the postoperative course, both serum pancreatic enzymes decreased rapidly from day 2 with no further significant differences between the two groups of patients.
C-reactive protein did not show a significant difference (P=0.188) on day 1 (HTK: 67.5±46.6 mg/L vs. UW 56.0±35.3 mg/L) (Figure 5).
After12 months, fasting glucose levels (HTK vs. UW: 5.42±1.29 vs. 5.54±1.03 mmol/L; P=0.616) and HbA1c (HTK vs. UW: 5.37±0.72% vs. 5.55±0.83%; P=0.259) were not significantly different between the two groups nor was the renal graft function (serum creatinine; HTK vs. UW: 133±50 vs. 139±59 mmol/L; P=0.602).
Acute Rejections
There were no significant differences in acute rejection rates between the two groups: 13 rejections were diagnosed in each group (27.1% and 27.7% in HTK and UW groups, respectively; P=1.000). Out of the 13 rejections in the HTK group, 7 were successfully treated with steroid bolus therapy, 5 patients were converted from CyAME to tacrolimus and there was one C4d positive rejection which was treated by steroids and increased baseline immunosuppression. Out of the 13 rejections in the UW group, 5 were steroid sensitive, 6 were converted to tacrolimus and two C4d positive rejections were treated, in one case with ATG and in the other case with increased baseline immunosuppression. As mentioned above, a total of 6 pancreas graft losses due to rejection were observed: 4 in the HTK group (with one subsequent kidney graft loss) and 2 in the UW group.
In our center, HTK has been used as the routine organ preservation solution in abdominal organ procurement since 1992. Gubernatis et al. [5] published the first clinical experiences from Hannover with HTK in liver transplantation with 14 patients in 1990. These results were confirmed by a controlled trial carried out by German researchers, showing no difference between primary non-function and survival after liver transplantation, even with a prolonged cold ischemic time of more than 15 hours [6]. In recent years HTK has been introduced in many transplant centers, mainly in Europe, demonstrating similar outcomes in post mortem [7, 8] and living donor liver transplantation [9] as well as in renal transplantation [10, 11]. On the contrary, UW is the standard preservation solution worldwide. Meanwhile, HTK is being used more often, probably due to lower costs [4] and because it gives equal clinical results in liver [12] and pancreas transplantation [1, 2, 3, 4]. Various concerns exist regarding its use in pancreas transplantation, especially when considering that UW was initially developed for experimental pancreas transplantation [13], whereas HTK was developed for cardioplegia [14]. The ongoing debate within German and Eurotransplant Foundation pancreas transplant centers is whether UW is the favored organ preservation solution for pancreas grafts. This debate commenced when pancreas grafts were no longer only transplanted locally, but were shipped to transplant centers in neighboring regions according to the Eurotransplant Foundation allocation rules, thus having a longer calculated cold ischemic time.
In our series all locally harvested pancreata were perfused with HTK whereas, out of nonlocal recovered grafts, only one-third were perfused with HTK and the others with UW solution. This fact might have an impact on the higher number of graft losses due to pancreatitis in the UW group. It should be noted that there was no significant difference in the cold ischemic time between the groups. Clinical experience and evaluation of HTK in pancreas transplantation is limited. There are only a few recently published studies [1, 2, 3, 4] including one with up-dated results [3]. Agarwal et al. [3] reported their experience with 78 pancreas transplantations with HTK comparing it to 10 grafts with UW perfusion as historical control. The group could show no differences in postoperative graft function in terms of serum amylase and fasting blood sugar with excellent patient and graft survival in the HTK group. The Pittsburgh group [1] reported its initial experience with HTK in pancreas transplantation with 16 patients compared to 17 patients in the UW group showing comparable clinical results of early graft function and survival rates. The multicenter study published by Englesbe et al. [4], which compared 36 patients transplanted using HTK and 41 patients transplanted using UW, demonstrated comparable outcome data. Nonetheless the HTK group did have significantly more acute rejections within the first 6 months; however, the authors attributed this to a change of protocol biopsies. Overall, these results are in line with our data, except that we did not find an increase of rejection rate in our patients.
All of these studies showed no significant differences in outcome and laboratory data, although comparison was limited due to different parameters such as cold ischemic times and donor age
Another useful marker of pancreas graft damage can be C-reactive protein [15]: Wullstein et al. showed that high peak serum CRP levels are associated with a higher risk of pancreas graft complications. In our study, we could not find any difference between HTK and UW in the risk of graft loss during the first postoperative week.
In addition, Potdar et al. [1] mentioned important subjective observations that HTKflushed pancreata appeared more edematous, and the venous effluent was much clearer during back-table preparation. These observations are concurrent with our own experience and that of others. Hesse et al. [16] could demonstrate that HTK-flushed grafts had more edema and a significant increase of wet/post perfusion weight after cold storage in an experimental porcine transplant model. The histological crosssections showed increased interstitial swelling without any parenchymal destruction, suggesting that this initial edema did not impair early graft function.
As a low volume organ [17], the pancreas is certainly more vulnerable to developing edema as compared to the liver or the kidney. The low viscosity of HTK requiring a larger perfusion volume, may lead to an added extracellular flush, hence resulting in edema. On the other hand, using high-volume perfusion - as initially suggested by Bretschneider [14] in order to achieve equilibration of interstitial and intracellular space - is not necessary. Abdominal organs can be safely preserved with a low total volume of 5-7 L of HTK. This also correlates with the clinical findings of the Englesbe group [4]. Especially in cases of prolonged donor operations, e.g. lung procurement, the flush volume should be reduced after the initial rapid flush in order to cool down the organs. The total time to completely achieve electrolyte equilibration requires 10 min of HTK perfusion, but, already within the first 4 minutes, sodium equilibration is accomplished [18]. In this study, no difference in cold ischemic time between HTK and UW could be demonstrated. In routine use, HTK offers several advantages such as easier handling (no additives) and generally lower costs. A lack of technical procurement skills might raise the risk of severe graft pancreatitis. Therefore, graft retrieval by experienced transplant surgeons would probably result in greater benefits and positive impact rather than the question of the use of UW or HTK solution.
In conclusion, HTK perfusion showed equal clinical results as compared to UW perfusion in clinical pancreas transplantation. In HTKperfused grafts, the peak lipase level was significantly higher and more edema could be observed without having any clinical impact on early graft function. From our clinical point of view, HTK can be safely used in pancreas preservation.