Keywords
Metallogenic; Carbonaceous; Isotopes; Genesis; Derivation; Precipitation
Introduction
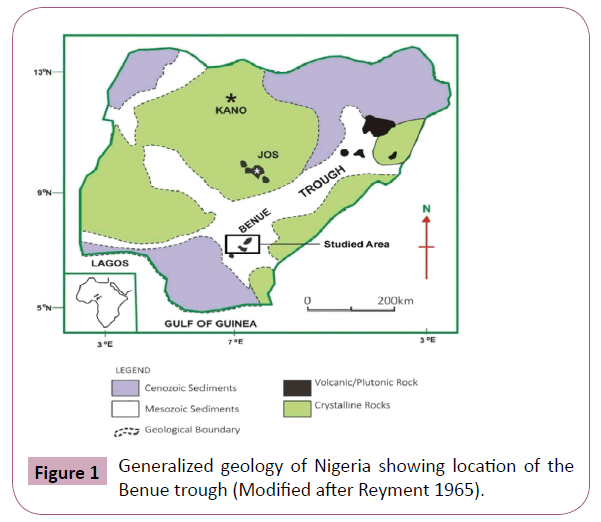
The Benue trough is a linear trough with a strike length of more than 700 km. It has a maximum width of about 160 km. in the southwest near the Gulf of Guinea but tapers to a mere 50 km in the northeast south of the Chad Basin. Figure 1 is the location map of the Benue Trough.
Figure 1: Generalized geology of Nigeria showing location of the Benue trough (Modified after Reyment 1965).
The lower Benue which forms the southwestern part of the trough is endowed with a cluster of base metal occurrences located at Ameri, Ameka, Akpatakpa, Enyigba and Isiagu. Orajaka studied the lead-zinc deposits at Enyigba, Ameri and Ameka and observed the abundant development of gangue and ore minerals in partially filled vugs and cavities. He suggested cavity filling processes of deposition from a telemagmatic source [1].
Olade on the basis of geology of mineralization and microthermometry proposed the deposition of the Benue Valley lead-zinc mineralization from basinal brines generated by abnormal geothermal heat produced during the intra-cratonic development of the Benue Trough [2]. Akande and Mucke favoured a basinal brine expulsion model for the origin of the Lower Benue sulfide deposits. Fatoye and Gideon gave a synopsis of the mineral resources in the Lower Benue Trough without any genetic implication for the mineral occurrences [3,4].
The stable Isotope ratio 13C/12C, 18O/16O as well as D/H have all been used in many ore studies to define their parameters of deposition [5-9]. In addition, Clayton et al. have used both δ13C and δ18O values of carbonate minerals to differentiate between marine and non-marine carbonate deposits [10,11].
Waters of diverse origin form the medium through which salts and base metals are transported prior to ore deposition. The use of hydrogen and oxygen isotopes in establishing the origin of such waters is based on the premise that natural waters of various sources e.g. meteoric, magmatic, formation, seawater, etc show systematic variations in their deuterium and 18O isotopic compositions.
Recent advances in ore deposit research have also shown that the geochemical nature and sources of the ore-forming fluids can best be determined when isotopic studies of carbon, oxygen, hydrogen and sulfur are carried out on the deposits. In view of the paucity of scientific information on the isotopic nature of the Lower Benue valley deposits, this study aims at providing additional data on the carbon and oxygen isotopic composition of pre-sulfide siderite, post-sulfide calcite and dolomite. The isotopic analysis of the hydrogen in water of fluid inclusions were also determined to assist in the interpretation of the history of the ore-forming fluids in the hope that this could be helpful in resolving the controversy that has surrounded the genesis of these hydrothermal vein deposits.
Geological setting
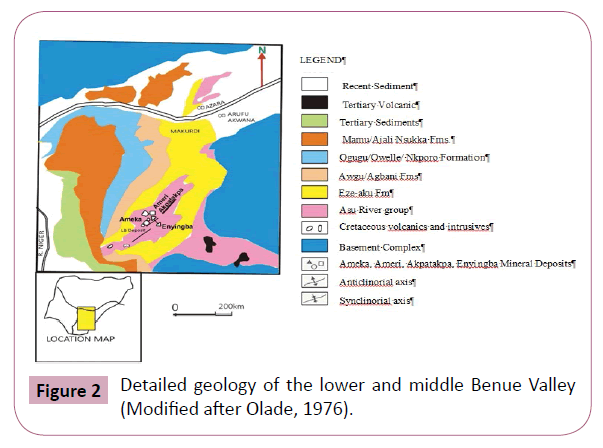
The Lower Benue basin occupies the deepest part of the trough where Cretaceous sediment infill amounts to about 5000 m of which 2000 m, belonging to the Albian Asu River Group, consists of unmetamorphosed black carbonaceous shales, siltstone and limestones including a minor underlying pyroclastic as shown in Figure 2.
Figure 2: Detailed geology of the lower and middle Benue Valley (Modified after Olade, 1976).
Arkosic sandstones underlying the shale and siltstones have been reported from the bottom of a borehole in the Abakaliki anticline but their precise nature, age and thickness are unknown [12,13]. The Albian sediments were deformed during the Cenomanian before the deposition of the overlying Turonian-Eze aku Formation consisting of alternating shale, limestone’s and sandstones [14]. This formation is overlain by the nearly 1000 m thick Senonian Awgu Formation. Tectonic deformation of the sediments in the Santonian produced numerous folds, faults and fractures.
Lead–zinc mineralization as open space filling is restricted exclusively to sheeted structures developed sometimes in an en-echelon pattern in the lower Cretaceous Albian sediments. These discordant and fault controlled ore deposits are relatively narrow, lenticular (3 m wide) or irregular. Their width may be up to 18 m whenever they occur as “pinches and swells” within numerous inter-connecting vugs which serves as open channels for circulating hydrothermal solutions [15]. The age of mineralization of these deposits is not known precisely. What is clear so far is that these deposits are found in Albian sediments. Secondly, they are epigenetic and occur in fractures that were reopened during tectonic brecciation that preceded the mineralization. In most of the mines, a complete sequence of the Cretaceous sediments is not preserved due to regional unloading and non-deposition. For example at Isiagu, the lead-zinc ores occur within folded Albian sediments in close proximity and at the same elevation with unmineralized Coniacian Awgu shales. There is therefore confusion as to whether to extend mineralization to the end of the Turonian since there was no sedimentary deposition in the mineral district during the Cenomanian.
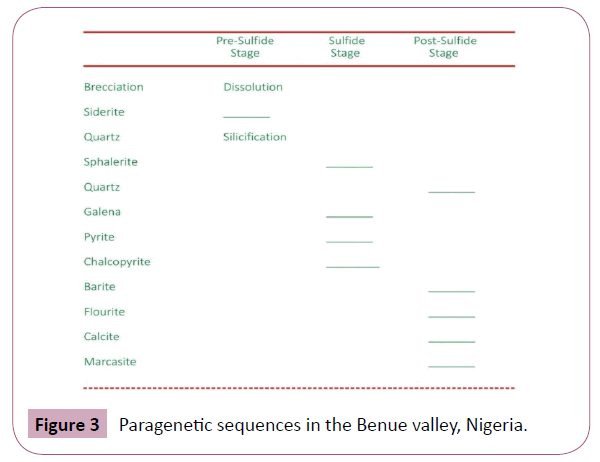
The mineralogy is simple consisting of sphalerite and galena as the major sulfide ores with sphalerite dominant in all the localities except Enyigba. Siderite is the main pre-ore mineral and it occurs as a massive crystalline aggregate with light brownish colour. It is the main gangue mineral which on oxidation changes to hematite which occurs as veinlets in foot-walls. Quartz, calcite, dolomite, and marcasite occur as post-sulfide minerals. Calcite and dolomite occurs as veins with very low dip and trending in a NW–SE direction. Chalcopyrite and Pyrite occur as minor sulfide accessories as shown in Figure 3.
Figure 3: Paragenetic sequences in the Benue valley, Nigeria.
Materials and Methods
Carbon and Oxygen isotopic compositions of calcite were analyzed by the techniques described in McCrea. While the isotopic compositions of siderite were determined by the techniques described in McCrea and modified according to the conversion factors of Friedman and O’Neil and by Rosenbaum and Sheppard [7,16,17].
In the Isiagu-Abakaliki mineral district sphalerite and galena are the ore minerals sampled while siderite, pyrite and quartz were collected as gangue minerals and the carbonaceous shales with bands of lateritised sandstone were collected as host rock. The carbonaceous shales are micaceous greyish to pinkish in colour with very gentle dips about 300 and usually intercalated with thin siltstone bands. The fresh samples were collected from open pits opened by private companies or by the natives. Some samples were also collected from old mine dumps.
Samples were sorted for mineralogical study of the silicate and gangue minerals. The preliminary separation of the ore minerals from the gangue were achieved by hand picking the coarsely crystalline ore. Mass spectrometric analysis to determine the isotopic composition of oxygen, carbon and hydrogen were carried out in the laboratories of the Centre de Recherhes Petrographiques et Geochimiques (C.R.P.G.-C.N.R.S.) Nancy, France [18].
The δD of water in fluid inclusions were analyzed by the technique described by Biegeleisen and Mayer and modified in Roedder and Heyl [19]. Isotopic compositions of samples are presented as per mil values relative to international standards. The hydrogen and oxygen isotope data are relative to Standard Mean Ocean Water (SMOW) while the carbonate values are relative to Pee Dee Belemnite (PDB).
Results and Discussion
Stable isotopes studies
Carbon, oxygen, and hydrogen form an important component of any environment. These elements have characteristic stable isotopes which serve as indicators of environmental conditions. The result of carbon and oxygen isotope measurements carried out on calcite, siderite, dolomite and carbonate in carbonaceous shale from the Lower Benue Pb-Zn deposits are presented in Table 1.
| Sample No. |
Locality |
Mineral/Rocks |
δ13c |
δ18o |
| AMK 03 |
Ameka |
Calcite |
-3.5 |
+17.8 |
| AMK 05 |
“ |
“ |
-3.2 |
+17.1 |
| AMK 07 |
“ |
“ |
-2.3 |
+17.8 |
| AMK 07b |
“ |
Dolomite |
-2.2 |
+16.4 |
| AMK 09 |
“ |
Carbonaceous shale Ct |
-3.3 |
+17.8 |
| AMK 17 |
“ |
Siderite |
-1.4 |
+14.5 |
| ENG 01 |
Enyigba |
Calcite |
-3.1 |
+17.5 |
| ENG 03 |
“ |
“ |
-3.1 |
+17.8 |
| ENG 04 |
“ |
“ |
-3.3 |
+17.0 |
| ENG 06 |
“ |
“ |
-2.7 |
+18.4 |
| ENG 06b |
“ |
Dolomite |
-2.9 |
+17.5 |
| ENG 09 |
“ |
Carbonaceous shale Ct |
-3.3 |
+18.5 |
| ENG 15 |
“ |
Siderite |
-1.1 |
+14.5 |
| AMR 01 |
Ameri |
Calcite |
-3.6 |
+17.6 |
| AMR 02 |
“ |
“ |
-3.1 |
+18.0 |
| AMR 03 |
“ |
“ |
-2.0 |
+17.7 |
| AMR 07 |
“ |
“ |
-2.3 |
+17.8 |
| AMR 08 |
“ |
Carbonaceous shale Ct |
-3.9 |
+17.7 |
| AMR 10 |
“ |
Calcite |
-5.3 |
+17.4 |
| AMR 10b |
“ |
Dolomite |
-4.5 |
+16.6 |
| ARU 09b |
Arufu |
Dolomite |
-2.0 |
+18.2 |
| ISH |
Isiagu |
Siderite |
-1.4 |
+15.4 |
Table 1: Carbon and oxygen isotopic composition of vein and country rock carbonates in the Benue Valley, Nigeria.
The δ13C values for pre-sulfide vein calcites in all the Lower Benue deposits range from -5.3 to -2.0 per mil (mean of -3.12 per mil). For dolomite, the carbon isotopic composition ranges from -4.5 to -2.0 per mil (mean of -2.9 per mil) while δ13C values for pre-sulfide siderite which is the first non-sulfide mineral to be deposited ranges from -1.4 to -1.1 per mil (mean -1.3 per mil). Isotopic measurements carried out on black carbonaceous shale hosting the deposit gave δ18O values ranging from -3.9 to -3.3 (mean -3.5 per mil). The δ18O isotopic composition for the calcites range from +17.0 to +18.4 per mil (mean +16.18 per mil) while that of dolomite ranges from +16.4 to + 17.5 per mil (mean +17.18 per mil). For siderite and carbonaceous shale the δ18O values are +14.5 to + 15.4 per mil (mean +14.8 per mil) and +17.7 to +18.5 per mil (mean +18 per mil) respectively.
The most striking features of the carbon isotopic data is the relatively narrow range exhibited by all the carbonates. The siderites are the most enriched and they have an extremely small range of composition with the other carbonate minerals. Calcite from the black carbonaceous shale country rocks is isotopically indistinguishable from the vein calcite and dolomite for both δ13C and δ18O. The relatively narrow range in the δ13C values of calcite from the different deposits indicates that the carbon isotopic compositions of the fluids probably varied within a similarly narrow range during the deposition of the calcite at each deposit and are genetically related. The dolomites are the most depleted in both δ13C and δ18O values.
The variation in isotopic composition may be due to lack of homogeneity in the bulk composition of the dolomites in contrast to the other carbonate species. There is a close similarity between the δ13C and δ18O values between the vein carbonate and the shale calcite suggesting that they probably shared a common source. The δ13C values of calcite exhibit a slightly larger spread than the corresponding δ18O values. The narrow spread of δ18O of the calcite may be controlled by the oxygen isotopic composition of the hydrothermal fluids.
Source of carbon
Studies have shown that the average δ13C value of marine limestone fluctuate between –3 and +3 per mil. The measured δ13C values in the vein carbonate species are similar, suggesting these come principally from sedimentary limestone sources. The δ13C values are also similar to the δ13C values of CO2 collected from geothermal areas with carbon isotopic composition ranging from –2 to –6 per mil. The Benue Trough being an intra-cratonic rift with a geothermal gradient could derive its carbon isotopic composition from this source. The isotopically lighter carbon in the black carbonaceous shales could have resulted from the oxidation of organic matter contained in the shale to produce the carbon enrichment in 12C. It should be noted that there is a general depletion in 13C in all post sulfide carbonates in contrast to the enrichment observed in pre-sulfide siderite δ13C values of -1.3 per mil. This variation can be due to several causes; however, it is possible that the CH4 content of the fluid decreased during precipitation of the post-sulfide carbonates. In view of the above it is possible that the precipitation of the Benue trough carbonates was dominantly derived from the oxidation of organic carbon with a possible small contribution from the dissolution of marine carbonates. The δD measurements of water in primary fluid inclusions in sphalerites and quartz are presented in Table 2.
| Specimen No. |
Locality |
Paragenesis |
Description |
δD‰ |
F.T.°C |
Salinity Eq. wt.
%NaCl |
| ISH 2 |
Isiagu |
Sulfide Stage |
Reddish brown sphalerite |
-28 |
- |
- |
| ISH 11 |
“ |
“ |
“ |
-19 |
130 |
21.2 |
| ISH 27 |
“ |
Post Sulfide Stage |
Milky white quartz encrusting sphalerite |
- 40+2(2) |
133 |
12.1 |
| AKP 22 |
Akpatakpa |
Sulfide Stage |
Massive sphalerite |
-34+-2(2) |
- |
- |
| AKP 18 |
“ |
“ |
Reddish brown sphalerite |
-22 |
153 |
21.2 |
| AKP 5 |
“ |
Post Sulfide Stage |
Crystalline quartz cross-cutting sphalerite |
-35 |
142 |
21.2 |
| ENG 2 |
Enyigba |
Sulfide Stage |
Reddish brown Massive sphalerite |
-15 |
149 |
21.2 |
| ENG 8 |
“ |
“ |
“ |
-17 |
- |
- |
| AMR 10 |
Ameri |
Sulfide Stage |
Reddish brown Massive sphalerite |
-13 |
156 |
21.2 |
| AMR 5 |
“ |
“ |
“ |
-19 |
- |
- |
| AMK 4 |
Ameka |
Sulfide Stage |
Reddish brown Massive sphalerite |
-14 |
-- |
- |
| AKF 3 |
Akwana |
Post Sulfide Stage |
Light Purple Fluorite |
-18 |
146 |
12.1 |
| AKF 7 |
“ |
“ |
Gray Fluorite |
-17 |
- |
- |
| ARU 7 |
Arufu |
Post Sulfide Stage |
Dark gray Fluorite |
-18 |
132 |
12.1 |
| ZUR 13 |
Zurak |
Sulfide Stage |
Black brownish sphalerite coexisting with galena |
-28 |
220 |
12.1 |
| ZUR 16 |
“ |
“ |
Red brownish sphalerite |
-23 |
- |
- |
| ZUR 6 |
“ |
“ |
Cubic galena |
-35 |
- |
- |
Table 2: Hydrogen isotopic composition of minerals in the Benue Valley.
The δD isotopic composition for the sphalerites in the lower Benue Trough ranges from -13 to -28 per mil. On the other hand, the hydrogen isotopic composition of post sulfide quartz which ranges from -34 to -40 per mil are markedly different from that of sphalerites. Carbon, Oxygen and Hydrogen are important components of any environment. These elements have characteristic stable isotopes which are useful as indicators of environmental conditions. The stable isotopes ratios 13C\12C 18O\16O as well D\H have all been used in many ore studies to define the parameters of ore deposition. Variations in D/H and 18O\16O ratios have been used by several workers notably Clayton et al and Craig as direct isotopic tracers of the sources of fluids that constitute hydrothermal solutions in sedimentary basins and geothermal system.
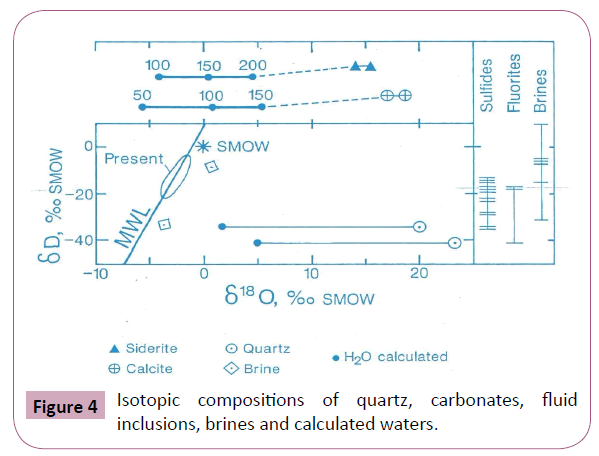
The isotopic data shows two patterns of hydrogen isotopic composition which can in general be related to the position of the minerals in the paragenesis. The early minerals (especially the sphalerites) are typically 10 to 20 per mil more enriched in D than the late cross-cutting quartz. In general, the sphalerites with the exception of those from Akpatakpa fall within the range -13 < δD < -28 per mil while the quartz exhibit δD isotopic composition between–35 < δD < -40 per mil. This shows that the sphalerites and the quartz were deposited from two distinct fluids. Ogundipe has also shown that there were chemical and thermal variations within the lower Benue. Figure 4 is a plot of the δD vs. δ18O isotopic composition of quartz, water, brines and carbonate vein minerals.
Figure 4: Isotopic compositions of quartz, carbonates, fluid inclusions, brines and calculated waters.
The data thus presented indicate that the H, O, salinity and homogenization temperature of the Benue metallogenic province is consistent with sedimentary basin formation fluids. These formation waters must be dominantly of meteoric origin; however, a significant seawater contribution could be present [19-24].
Conclusion
From the data on the carbon isotopic variations it is probable that the carbon that participated in the precipitation of the vein carbonate in Benue valley was dominantly derived from the oxidation of organic carbon with a possible small contribution from the dissolution of marine carbonate.
Finally, the variation in the δD isotopic composition probably reflects that different “packets” of fluids came from different depths and lithological units within the basin.
Acknowledgements
I acknowledge the assistance of Professors M. A. Olade and S. M. F. Sheppard who were my supervisors at the University of Ibadan, Nigeria and Centre de Recherché Petrographiques Geochimiques, (C.R.P.G) Nancy, France respectively. Study leave for the research was granted by the Federal Polytechnic, Ado-Ekiti and funded by the European Economic Community (EEC) Brussels.
References
- Akande SO, Zentilli M, Reynolds PH (1989) Fluid inclusion and stable isotope studies of the Pb-Zn-fluorite-barite mineralization in the lower and middle Benue Trough, Nigeria. Mineralium Deposita24: 183-191.
- Charef A, Sheppard SMF (1987) Pb-Zn mineralization associated with diapirism: Fluid inclusion and stable isotope (H, C, O) evidence for the origin and evolution of the fluids at Fedj-EL-Adoum, Tunisia. Chem Geol 61: 113-134.
- Clayton RN, Friedman I, Graf DZ, Mayeda TK, Meents WF, et al. (1966) The origin of saline formation waters. J Geophys Res 71: 3869-3882.
- Craig H (1966) Isotopic composition and origin of the red sea and salton sea geothermal brines. Science 154: 1544-1548.
- Fatoye FB, Gideon YB (2013) Geology and mineral resources of the lower Benue Trough, Nigeria. Adv Appl Sci Res 4: 21-28.
- Friedman I, O’Neil JR (1977) Compilation of stable isotope fractionation factors of geochemical interest. USGS 440: 12.
- Hitchton B, Friedman I (1969) Geochemistry and origin of formation waters in the western Canada sedimentary basin–I. Stable isotopes of hydrogen and oxygen. Geochim Cosmochim Acta 33: 1321-1349.
- Keith ML, Weber JN (1964) Carbon and Oxygen isotopic composition of selected limestone and fossils. Geochim Cosmochim Acta 28: 1787-1816.
- Maurin JC, Lancelot JR (1987) Origine des mineralisations de Pb-Zn de la vallez, Benoue (Nigeria) d’aprÃÆÃââââ¬ÃÅÃÆââ¬Å¡ÃâÃÂs la composition en Pb des galÃÆÃââââ¬ÃÅÃÆââ¬Å¡ÃâÃÂnes et de l’encaissant. Mineral Deposita 22: 99-108.
- McCrea JJ (1950) On the isotopic chemistry of carbonates and a paleotemperature scale. J Chem Phys 18: 849-857.
- Murat RC (1970) Stratigraphy and paleontology of the cretaceous and lower territory in Southern Nigeria. In: African Geology, Ibadan Printing Press, Nigeria.
- Nwachukwu SO (1972) The tectonic evolution of the southern portion of the Benue Trough, Nigeria. Geol Mag 109: 411-419.
- Ogundipe IE, Obasi RA (2016) Geology and mineralization in the Albian sediments of the Benue Trough, Nigeria. British J Earth Sci 4: 1-15.
- Ogundipe IE (2017) Thermal and chemical variations of the Nigerian Benue trough lead-zinc-barite-fluorite deposits. J. Afr Earth Sci 132: 72-79.
- Olade MA (1976) On the genesis of lead-zinc deposits in Nigeria’s Benue rift (Aulacogen), a re-interpretation. J Min Geol 13: 20-27.
- Orajaka S (1975) The geology of Enyigba, Ameri and Ameka Pb-Zn nodes, Abakaliki division, Eastern Nigeria-A reconnaissance. Jour Min Geol 2: 65-70.
- Ohmoto H (1986) Stable isotope geochemistry of ore deposits. In: Valley JW, Taylor HP, O’Neil JR (eds.) Stable isotopes in high temperature geological processes. Reviews in Mineralogy 16: 491-556.
- Reyment C (1965) Aspects of geology of Nigeria. Ibadan University Printing Press, Nigeria.
- Roedder E, Heyl AV (1968) Environment of ore deposition at the Mex-Tex deposits, Hansburg District New Mexico from studies of fluid inclusion. Econ Geol 63: 336-348.
- Rosenbaum J, Sheppard SMF (1986) An isotopic study of siderites, dolomites and ankarites at high temperatures. Geochim Cosmochim Acta 50: 1147-1150.
- Sheppard SMF (1977) Identification of the origin of ore-forming solutions by the use of stable isotopes. In: Volcanic Processes in Ore Genesis. Institution of Mining and Metallurgy and Geological Society of London, UK.
- Sheppard SMF (1984) Stable isotope studies of formation waters and associated Pb–Zn hydrothermal ore deposits. In: Durand B (ed.) In Thermal Phenomena in Sedimentary Basins. Editions Technip, Paris.
- Taylor HP (1974) The application of oxygen and hydrogen isotope studies to problems of hydrothermal alterations and ore deposition. Econ Geol 69: 843-883.
- Taylor HP (1979) Oxygen and hydrogen isotope relationships in hydrothermal mineral deposits. In: Barnes HL (ed.) Geochemistry of hydrothermal ore deposits (2nd edn.). John Wiley and Sons, NY, USA.