Original Article - (2017) Volume 18, Issue 3
Departments of 1Pancreatic Surgery, 2Pancreatic Cancer Institute, and 3Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, P.R. China
Received March 12th, 2017-Accepted April 12th, 2017
Background The predictive significance of O-6-methylguanine-DNA methyltransferase promoter methylation for patients with neuroendocrine tumor and treated with alkylating agent chemotherapy remains controversial. This meta-analysis describes whether O-6- methylguanine-DNA methyltransferase expression and promoter methylation could help predict neuroendocrine tumor response to alkylating agent chemotherapy. Methods We conducted a systematic search in PubMed, EMBASE and the Cochrane library and identified articles describing a relationship between O-6-methylguanine-DNA methyltransferase status and patients with neuroendocrine tumor response to alkylating agent chemotherapy. Results Ten articles were included in our analysis. The O-6-methylguanine-DNA methyltransferase deficiency rate measured by immunohistochemistry was similar to the O-6-methylguanine-DNA methyltransferase promoter methylation rate measured by pyrosequencing. The O-6-methylguanine-DNA methyltransferase deficiency rate was higher in patients with pancreatic neuroendocrine tumor than in patients with gastrointestinal- neuroendocrine tumor (p<0.05). Neuroendocrine tumor with O-6-methylguanine-DNA methyltransferase deficiency/promoter methylation had a significantly longer progression-free survival when treated with alkylating agent chemotherapy, regardless of detection method: immunohistochemistry (hazard ratios 0.39, 95% confidence intervals 0.17–0.89) or pyrosequencing (hazard ratios 0.33, 95% confidence intervals 0.19–0.56). Also, neuroendocrine tumor with O-6-methylguanine-DNA methyltransferase deficiency had a better objective response rate to alkylating agent chemotherapy (by immunohistochemistry p=0.01, by pyrosequencing p=0.02). The subgroup analysis showed no significant association between O-6- methylguanine-DNA methyltransferase status and objective response rate in pancreatic neuroendocrine tumor patients. Conclusion O-6-methylguanine-DNA methyltransferase deficiency/promoter methylation predicts a favorable response to alkylating agents in neuroendocrine tumor patients, regardless of the O-6-methylguanine-DNA methyltransferase detection method. However, the predictive roles of O-6-methylguanine-DNA methyltransferase deficiency in pancreatic neuroendocrine tumor patients need further assessment.
Alkylating Agents; Meta-Analysis, Neuroendocrine Tumors; O(6)-Methylguanine-DNA Methyltransferase
Neuroendocrine tumors (NETs) are a group of rare and heterogeneous tumors. According to the National Cancer Institute Surveillance, the incidence rate of NET is 0.43 patients per 100000 persons, which is double than that seen 20 to 30 years ago [1] . Based on the primary tumor site, NETs can be divided into subgroups, such as pancreatic NET (pNET) and gastrointestinal NET (GI-NET). In China, pNET is the most common form, which accounts for 49.8% of the total patients with NET in the country [2]. Alkylating agent chemotherapy, particularly temozolomide-based chemotherapy, is a standard treatment for certain NETs, which has exhibited a promising overall response rate in the treatment of NET [3, 4].
Temozolomide produces anti-tumor activity by inducing DNA methylation at the O6 and N7 positions of guanine, which leads to DNA mismatch and tumor cell death [5, 6]. DNA repair enzyme O-6-methylguanine DNA methyltransferase (MGMT) is crucial for genome stability as it repairs DNA mismatch and transcription errors [5]. MGMT removes a mutagenic alkyl group from O6-guanine and transfers it to an active cysteine. Studies have shown that diminished MGMT expression increases the carcinogenic risk in mice exposed to alkylating agents [7], whereas high MGMT expression counteracts the therapeutic effect of alkylating agents, thus contributing to chemoresistance [8].
Loss of MGMT expression in high-grade gliomas is predictive of improved survival with temozolomide-based therapy [9, 10]. However, the role of MGMT methylation status in predicting the response to alkylating agents in patients with NET is still controversial. And we aimed to perform a meta-analysis of recent literature to elucidate the relationship between the predictive efficacy of MGMT expression and the response to alkylating agent chemotherapy in patients with NET.
Literature Search Strategy
We searched PubMed, Cochrane Library, and Web of Science for articles published up to December 12, 2016. The following keywords were used in our search strategy: (“O-6-methylguanine-DNA methyltransferase” OR “MGMT”) AND (temozolomide OR [8-carbamoyl-3- methylimidazo (5,1-d) -1,2,3,5-tetrazin-4 (3H) one] OR methazolastone) AND (Neuroendocrine neoplasm[Mesh]). We limited our search to studies written in English. The reference lists of the selected articles were searched to ensure that no studies were overlooked.
Selection Criteria
The meta-analysis included studies that met the following standards: (1) all patients with NET were diagnosed by histopathology; (2) the study reported data of MGMT expression levels or MGMT promoter methylation status; (3) the results were part of an original analysis; and (4) if the same patient population was used in several publications, then only the most complete study was included in the meta-analysis. The exclusion criteria were as follows: (1) publication type as abstract; (2) studies focusing on the value of MGMT co-expression and other factors rather than MGMT expression levels or MGMT promoter methylation status; and (3) studies without hazard ratios (HRs) or 95% confidence intervals (CIs), or without a Kaplan-Meier curve to calculate these data.
Data Extraction
Each article was reviewed independently by two authors. If differences in opinion arose between these two authors, then the articles were discussed with a third author. The selected articles were assessed according to “The Newcastle-Ottawa Scale for assessing the quality of non-randomized studies in meta-analyses” [11]. The following data were collected from each study: first author’s name, country, publication year, number of patients, primary location of NET, methodology of MGMT analysis, and objective response rate and progression-free survival (PFS) with HR and 95% CI. If the prognostic data were not directly expressed, we obtained the data from Kaplan-Meier curves (using Engauge Digitizer version 4.1) and calculated the HR and 95% CI by previously reported methods [12].
Statistical Analysis
Based on immunohistochemistry cut-off values used in each study, we categorized the expression of MGMT as either “deficient” or “intact”. The MGMT promoter methylation was determined as “methylation” or “non-methylation” by pyrosequencing or quantitative methylation-specific PCR. The end-points were objective response rate and PFS; and the association between MGMT and clinical outcome was evaluated by using the HR of negative/methylation MGMT status over positive/non-methylation MGMT status and the 95% CI. Multivariate Cox proportional hazards models were applied for this purpose. For patients with a positive/ non-methylation status, a HR greater than one with a 95% CI that did not overlap with one implied a good predictive value, whereas an HR lower than one with a 95% CI that did not overlap with one indicated a poor outcome in survival. An HR of one indicated a lack of association between MGMT expression and clinical prognosis. All data were synthesized by Review Manager (version 5.2). The Mantel-Haenszel test was used to test significance; a p<0.05 was considered statistically significant. Statistical heterogeneity was assessed by the visual inspection of forest plots, by performing the χ2 test, and by calculating the I2 value. Significant heterogeneity across studies was indicated by p<0.1 or I2>50% and a random-effects model was performed to calculate the pooled estimate; otherwise, the heterogeneity is not significant and a fixed-effects model was applied.
Characteristics of Studies
A flow chart of the study selection criteria is shown in Figure 1. Twelve articles with a total of 835 patients with NET were chosen based on the eligibility criteria; all studies tested MGMT expression or MGMT promoter methylation status. One article was excluded because it involved neuroendocrine cancer(NEC) patients [13]. One article was excluded because of unavailable data on MGMT expression level [14]. Thus ten articles were included in expression analysis. Seven articles with 519 patients with NET included objective response rate data [15, 16, 17, 18, 19, 20, 21]. Four articles with 289 patients with NET included PFS data [15, 16, 19, 21]; Two of these directly indicated the HR and 95% CI for PFS, and the remaining two studies did not include the HR and 95% CI, which we estimated from Kaplan- Meier curves. The patient characteristics and study quality levels are listed in Table 1. Three articles have no predictive data about MGMT expression in patients with NET treated with alkylating agents [22, 23, 24].
MGMT Expression in Patients with NET
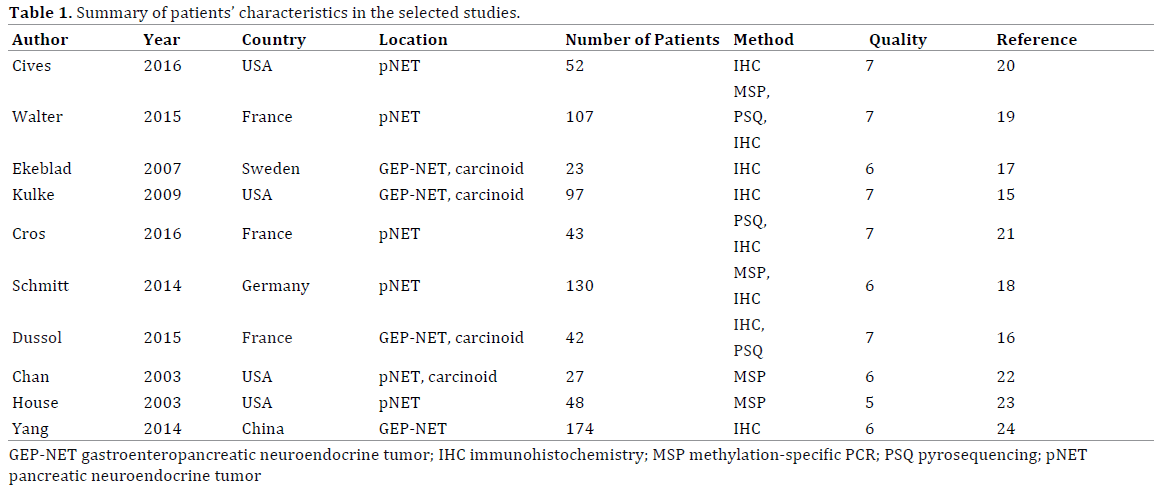
The MGMT expression data are shown in Table 2. Seven studies used the immunohistochemistry method for MGMT analysis and three applied pyrosequencing method. In immunohistochemistry subgroup, the MGMT expression level was evaluated according to the intensity or positive percentage of the staining. From these seven studies, three defined MGMT deficiency as complete absence of staining in all tumor cells [15, 16, 20], two described MGMT deficiency as a cut-off value of 10% [17, 19], and three defined MGMT status by using unique score systems. In pyrosequencing method subgroup, a threshold of 7–8% was used to classify the patients in “methylation” and “non-methylation” categories [16, 19, 21]. For NETs of all primary sites, the MGMT methylation rate detected by methylation-specific PCR and pyrosequencing was 12.7% and 24.8%, respectively. The MGMT deficiency rates were 27.3% for an immunohistochemistry cutoff value of 0% and 35.7% (range: 33–43.5%) for an immunohistochemistry cut-off value of 10%.
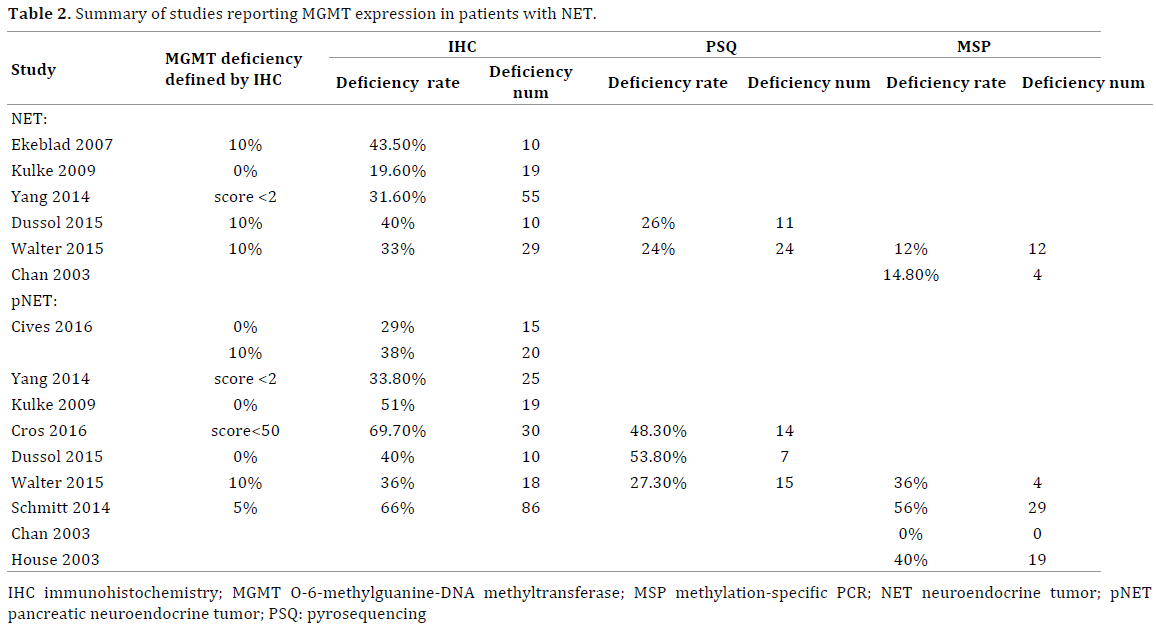
In the pNET subgroup, the MGMT deficiency rates were 35.9% (range: 36–38%) for an immunohistochemistry cut-off value of 0% and 32.1% (range: 29–51%) for an immunohistochemistry cut-off value of 10%. Further, the MGMT promoter methylation rates were 31.7% (range: 0–56%) by methylation-specific PCR and 37.1% by pyrosequencing. Although the MGMT deficiency rate indicated by methylation-specific PCR was lower than that indicated by pyrosequencing, the MGMT deficiency rates measured by the three methods of MGMT analysis were not significantly different (p>0.05).
Four articles included data for the GI-NET subgroup. Two studies applied the pyrosequencing method and found MGMT promoter methylation rates of 12.5% and 24%. Three studies applied the immunohistochemistry method with a cut-off value of 0% and found MGMT deficiency rates in the range of 0 to 27.7%. The MGMT deficiency rate measured by the immunohistochemistry method is significantly lower in the GI-NET subgroup than in the pNET subgroup (p<0.05).
Relationship between MGMT Promoter Methylation Status and PFS of Patients with NET Treated with Alkylating Agent Chemotherapy
The patient survival data are shown in Table 3. A total of 289 patients with NET were treated with alkylating agents such as temozolomide. The results of the MGMT status and the PFS of patients with NET are shown in Figure 2. The meta-analysis based on the immunohistochemistry method indicated that patients with MGMT deficiency had a significantly longer PFS when treated with alkylating agent chemotherapy (Figure 2a, average HR = 0.39, 95% CI 0.17–0.89). Similarly, the meta-analysis for studies that applied the pyrosequencing method indicated that the PFS is longer in patients with MGMT promoter methylation than in patients with MGMT non-methylation (Figure 2b; average HR = 0.33, 95% CI 0.19–0.56).
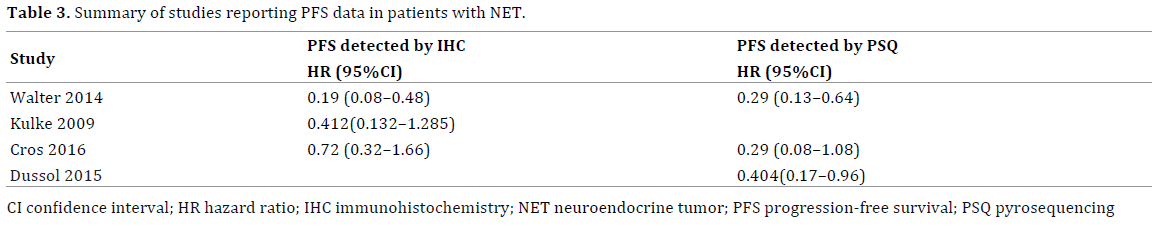
Figure 2. Forest plot showed the fixed effect model of the hazard ratio with 95% confidence intervals estimates of O-6-methylguanine-DNA methyltransferase expression and PFS in patients with neuroendocrine tumor treated with alkylation agents. (a). O-6-methylguanine-DNA methyltransferase detected by immunohistochemistry method and (b). pyrosequencing method.
Relationship between MGMT Promoter Methylation Status and Objective Response Rate of Patients with NET Treated with Alkylating Agent Chemotherapy
Seven articles provided objective response rate data for the treatment of NET (Supplemental Table 1). The meta-analysis results for the MGMT status and the objective response rate are shown in Figure 3. For the immunohistochemistry method, the meta-analysis indicated a significantly lower objective response rate in patients with intact MGMT compared to patients with MGMT deficient when treated with alkylating agent chemotherapy (average OR 7.21, p=0.01). Similarly, for the pyrosequencing method, the objective response rate was higher in patients with MGMT methylation than in patients with intact MGMT.
Figure 3. Forest plot showed the fixed effect model of the risk ratio with 95% confidence intervals estimates of O-6-methylguanine-DNA methyltransferase expression and objective response rate in patients with neuroendocrine tumor treated with alkylation agents. (a). O-6-methylguanine-DNA methyltransferase status detected by immunohistochemistry method and (b). pyrosequencing method.
Six articles provided objective response rate data for the treatment of pNET (Supplemental Table 1). The metaanalysis results for the MGMT status and the objective response rate are shown in Figure 4.The meta-analysis for either the immunohistochemistry or pyrosequencing methods showed no relationship between MGMT and objective response rate of patients with pNET treated with alkylating agent chemotherapy.
Figure 4. Forest plot showed the fixed effect model of the risk ratio with 95% confidence intervals estimates of O-6-methylguanine-DNA methyltransferase expression and objective response rate in patients with pancreatic neuroendocrine tumor treated with alkylation agents. (a). O-6-methylguanine-DNA methyltransferase detected by immunohistochemistry method and (b). pyrosequencing method.
Two articles provided objective response rate data for the treatment of GI-NET; however, the number of patients was too limited, thus we did not perform a GI-NET subgroup meta-analysis.
This study is the first meta-analysis focused on the association between MGMT status and the response of patients with NET to alkylating agent chemotherapy, particularly temozolomide-based chemotherapy. We aimed to solve three controversial questions regarding the treatment of NET: whether MGMT methylation is predictive of NET response to alkylating agent chemotherapy, whether the MGMT expression rate is different between pNET and GINET, and whether methodology of MGMT analysis influences the MGMT expression rate. In this meta-analysis, we showed that MGMT deficient in temozolomide-treated patients, as detected by immunohistochemistry and pyrosequencing, is associated with higher objective response rate and longer PFS. Further, we determined that the MGMT deficiency rate is higher in patients with pNET than in patients with GINET. We also found that the detection of MGMT status by immunohistochemistry, pyrosequencing, or methylationspecific PCR produces consistent results.
The predictive value of MGMT for NET remains controversial; a reason for this is the primary tumor location. Because NETs are clinically and biologically heterogeneous, it is increasingly clear that pNET are biologically distinct from GI-NET and carcinoid tumors [25, 26]. Compared to other NET, pNET shows higher objective response rate to cytotoxic chemotherapy [27, 28]. Walter et al. [19] found no difference in MGMT deficiency rate between GI-NET and pNET. MGMT promoter methylation is rarely detected in ileal NET but are found in other digestive segments in up to 25% of all patients [29]. Kulke et al. [15] found that MGMT deficiency, as measured by immunohistochemistry, is more common in pNET than in carcinoid tumors. In our meta-analysis, we found that the MGMT deficiency rate, detected by both immunohistochemistry and pyrosequencing, is higher in pNET than in GI-NET. However, the small number of patients with GI-NET may have influenced this result. Thus, more studies on GI-NET are needed to indicate with certainty whether MGMT deficiency has a predicting role in the clinical outcome of patients with GI-NET and patients with pNET after treatment with temozolomide -based therapy.
The different techniques used to assess MGMT status also contribute to the controversy behind the predictive value of MGMT for NET. MGMT deficiency can be assessed by three methods: immunohistochemistry to analyze MGMT expression, or methylation-specific PCR or pyrosequencing to analyze MGMT promoter methylation [30]. Although immunohistochemistry is the most convenient technique, it lacks standardization and reproducibility; thus, methylation-specific PCR and pyrosequencing have been explored as alternatives to assess MGMT status. Although MGMT promoter methylation is difficult or impossible to assess in fine-needle aspiration (FNA) biopsies when the tumor cell count is too low, pyrosequencing is more sensitive than methylation-specific PCR for the detection of MGMT promoter methylation in flash frozen paraffin-embedded tissue samples [31]. Small and heterogeneous cohorts applied different methods with various standards [17]. Retrospective studies of MGMT promoter methylation by pyrosequencing in patients with glioblastoma have consistently shown that patients with MGMT promoter methylation benefit from temozolomide treatment [9]. Until recently, there had been a paucity of information regarding NET analyzed by methylationspecific PCR and pyrosequencing for MGMT promoter methylation and by immunohistochemistry for MGMT expression. Both PFS and OS were prolonged in patients with MGMT deficiency/methylation after the first therapy with alkylating agents (temozolomide, dacarbazine, and streptozocin). Our study showed that the MGMT deficiency rate determined by immunohistochemistry and the MGMT promoter methylation rate determined by pyrosequencing are similar, and both methods of MGMT analysis can efficiently predict treatment response to alkylating agent chemotherapy in patients with NET. Walter et al. [19] compared immunohistochemistry, methylation-specific PCR, and pyrosequencing for the detection of MGMT status in patients with NET and found no difference in MGMT deficiency rates. Cros et al. [21] found that increased promoter methylation is associated with MGMT deficiency; Our finding provides important information on the role of immunohistochemistry, methylation-specific PCR and pyrosequencing in MGMT analysis, highlighting the need for prospective, randomized studies to address these associations.
Grading may influence our results. Researchers nowadays agree that treatment options and prognosis of NET are greatly influenced by pathologic differentiation [28]. Welin et al. [13] reported that temozolomide-based chemotherapy could induce partial response in highly proliferative, poorly differentiated carcinomas. Civeset al. [20] showed no correlation between tumor grade, mitotic rate or Ki-67 labeling index, and tumor response to capecitabine and temozolomide (CAPTEM). This finding suggests that the efficiency of alkylating agent chemotherapy against NET may relate with Ki-67 scoring. However, due to the limited information and number of patients available, we were unable to divide data by grade subgroups.
Studies found three main DNA repair mechanisms are involved in temozolomide resistance: MGMT repair, DNA mismatch repair, and the poly(ADP)-ribose polymerase (PARP) pathway [32]. The primary mechanism of resistance to temozolomide is directly related to high MGMT expression, whereas the secondary mechanism is related to the DNA mismatch repair system in MGMTlacking cells [33]. The third mechanism depends on the PARP pathway, which may partly explain why not all patients with MGMT deficiency respond to temozolomidebased chemotherapy. Zhang et al. [34] showed that temozolomide-induced DNA damage and tumor cell death require a functional DNA mismatch repair system in addition to low MGMT expression. Moreover, Cros et al. [21] showed in their study that none of the low MGMT expression patients with non-response to temozolomide had a microsatellite instability phenotype. Further studies are needed to elucidate the relationship between MGMT status and other predictive biomarkers (e.g. microsatellite instability) for NET treatments.
To conclude, our results showed that patients with NET with MGMT deficiency/promoter methylation are more likely to have a promising response to alkylating agent chemotherapy, immunohistochemistry method or pyrosequencing method did not influence the result. However, the predictive role of MGMT is not significant in pancreatic patients with NET. We expect that further studies will apply the predictive value of MGMT to plan clinical treatments.
This work was supported by grants from the National Natural Science Foundation of China (81472670, 81172005, 81402397, 81402398, and 81172276), the National Natural Science Foundation of Shanghai (14ZR1407600), the“Yang-Fan”Plan for Young Scientists of Shanghai (14YF1401100), and the PhD Programs Foundation of Ministry of Education of China (20110071120096). The funding agencies had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
GHL and LL contributed to the literature searching, WWQ and WCT reviewed the searching results. GHL and ZSR wrote the manuscript. GHL, LL, and XHX performed the statistical analyses. YXJ and NQX participated in study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Heli Gao and Liang Liu contributed equally to this work.
The authors have declared that no competing interests exist.