- (2011) Volume 12, Issue 2
Anastasios T Dimou1, Konstantinos N Syrigos2, Muhammad Wasif Saif3
1Department of Pathology, Yale University School of Medicine. New Haven, CT, USA.
2Oncology Unit, Third Department of Medicine, University of Athens. Athens, Greece.
3Columbia University College of Physicians and Surgeons and Pancreas Center, New York Presbyterian Hospital. New York, NY, USA
Pancreatic adenocarcinoma has repetitively proved refractory to chemotherapy and biologic compounds with only a few drugs offering limited benefit. A number of novel regimens has been tested in phase I trials and reported recently in the 2011 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium. Specifically, a novel mitogen-activated protein kinase kinase (MEK) inhibitor, a connective tissue growth factor inhibitor, a coagulase factor VIIa inhibitor, as well as adenovirus delivery of herpes simplex virus thymidine synthase gene followed by anti-herpetic treatment have all proven to be safe in pancreatic cancer patients. Phase II trials will provide further evidence whether they can become clinically relevant in the future.
adverse effects; Clinical Trials, Phase I as Topic; Mitogen-Activated Protein Kinase Kinases; Pancreatic Neoplasms
CTGF: connective tissue growth factor; HSV: herpes simplex virus; MAPKK, MEK: mitogen-activated protein kinase kinase
Prognosis for pancreatic adenocarcinoma remains dismal [1]. In the metastatic setting, gemcitabine and erlotinib treatment results in a median overall survival of 6 months [2] whereas recently, the combination of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) has proven superior to the gemcitabine-erlotinib regimen at the cost of significant toxicity [3]. Thus, taking into account the shortage of effective compounds, development and testing of novel agents is necessary.
KRAS is a small GTPase bound to the cell membrane that drives major oncogenic pathways in human cancer. In addition, KRAS is mutated and constantly active in the vast majority of pancreatic adenocarcinomas [4] rendering a rationale for inhibition of that particular pathway at a level downstream of KRAS. Mitogenactivated protein kinase kinase (MAPKK or MEK) is an appealing target in this regard. Desmoplasia is another property of pancreatic adenocarcinoma that is responsible for resistance to existing treatments, since systemic compounds find it hard to penetrate through the thick fibrotic stroma [5]. Hence, designing treatment strategies that reduce tumor stroma emerges as an interesting approach. Anti-angiogenesis drugs have failed to prove effective in clinical trials illustrating the complex biology of tumor angiogenesis in pancreatic cancer [6]. It has been shown that anticoagulation can exert some anti-angiogenic properties in pre-clinical models providing an additional strategy of targeting tumor vasculature [7]. Finally, gene therapy might be an alternative for clinical trial design, given the emerging technology of gene delivery and engineering of tumor genomes.
Addition of MEK Inhibition to Standard Gemcitabine Treatment in Pancreatic Cancer
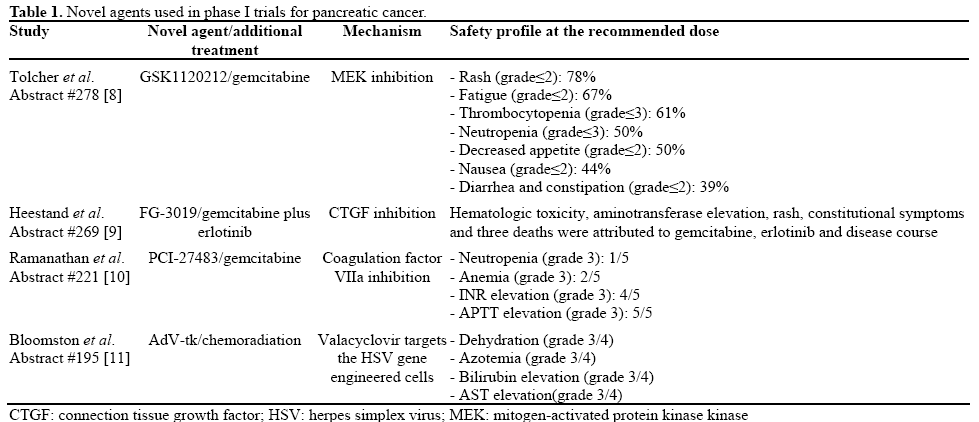
GSK1120212 is a reversible and specific inhibitor of MEK. Toicher et al. [8] (Abstract #278)have studied the safety profile, pharmacokinetic properties and potential efficacy of this compound in combination with gemcitabine in a panel of advanced solid tumors in the context of a phase Ib trial. Eight patients with pancreatic cancer were included in the studied population. Maximal tolerated dose was defined to be the combination of 2 mg of GSK1120212 given orally once daily with 1000 mg/m2 given on days 1, 8 and 15 every 28 days. Febrile neutropenia, aminotransferase elevation and uveitis limited further increase in dosage. Pneumonitis, febrile neutropenia and dyspnea were attributed to treatment on five occasions among 16 serious adverse events that were observed. Hematologic and gastrointestinal toxicity, as well as rash and constitutional symptoms (fatigue, loss of appetite), were the most frequent adverse events reported. There was no pharmacokinetic interaction between gemcitabine and GSK1120212. Interestingly, one patient with pancreatic cancer achieved a partial response and another 3 patients experienced stabilization of their disease for several months.
Connective Tissue Growth Factor is a Candidate Target in Pancreatic Cancer
FG-3019 is a monoclonal antibody against connective tissue growth factor (CTGF) which was tested in a phase I study by Heestand et al. [9] (Abstract #269). The experimental drug was administered every 2 weeks at 4 dose levels: 3, 10 and 15 mg/m2. After the first dose, erlotinib and gemcitabine were added. Seven severe adverse events and three additional adverse events including rash, hematologic toxicity and aminotransferase elevation were reported after gemcitabine and erlotinib were administered. Toxicity did not seem to be related to FG-3019. On the contrary, it correlated with the known spectrum of gemcitabine or erlotinib toxicity and the natural history of the disease. In this context, three deaths from sepses, suicide and tumor progression were reported among the severe adverse events. Patients progressed after a median time of 3.7 months and had a median survival of 9.4 months. Since a maximal tolerated dose was not defined, more patients were accrued to receive 25 mg/m2 FG-3019.
Anti-Coagulation as a Possible Anti-Angiogenesis Strategy
Ramanathan et al. [10] (Abstract #221) have studied the safety profile of PCI-27483, a small inhibitor that inhibits the coagulation factor VIIa in a selective fashion. They administered this compound in combination with gemcitabine in patients with advanced pancreatic cancer in the context of a phase I trial. Gemcitabine was administered at a fixed dosage of 1,000 mg/m2 on days 1, 8 and 15 every 4 weeks, whereas for the experimental drug, dosage was escalated in each patient in a three step fashion: 0.8, 1.2 and 1.8 mg/kg over the course of 4 to 8 weeks administered subcutaneously twice daily. An INR of 3 measured 2 hours after the injection of PCI-27483 was targeted as a surrogate for adequate anti-coagulation. Among the 5 evaluable patients of the study, grade 3 hematologic toxicity was observed in 3 patients whereas all the patients experienced a grade 3 elevation of INR, APTT or both. Four out of five patients were able to stabilize their disease for at least 4 months. The dose of PCI-27483 recommended for phase II trials is 1.2 mg/kg.
Anti-Herpetic Treatment Following Integration of a Herpes Simplex Virus (HSV) Gene in Pancreatic Cancer Cells
Bloomston et al. [11] (Abstract #195) performed a phase I study where they delivered an adenovirus vector containing HSV thymidine synthase gene in patients with locally advanced pancreatic cancer via endoscopic ultrasound or externally under computerized tomography guidance. Subsequently they treated their patients with the anti-herpetic drug valacyclovir for 14 days following the adenoviral delivery. Finally, patients received 5-FU based chemoradiation following the standard protocol and beginning from week 3. A total of 12 patients were accrued and assigned to 4 different dose levels of the adenoviral vector ranging from 3x1010 to 1012 vector particles. Although a number of grade 3 or 4 toxicities were observed including dehydration, azotemia, bilirubin and aminotransferase elevation, no dose limiting adverse events were reported. Efficiency of the protocol was promising with median overall survival being 12.2 months and 2 patients achieving a partial response (Table 1).
MEK inhibition has been tested before in pancreatic and other cancers using a different compound, CI-1040 but proved inefficient [12]. GSK1120212, is a novel MEK inhibitor that has unique pharmacokinetic properties and a broader therapeutic window than previous MEK inhibitors [13]. It might therefore prove more effective in phase II studies. FG-3019 has been tested before in a phase I study in patients with diabetes and albuminuria [14]. Likewise to the current study by Heestand et al., it was well tolerated by patients in the non-oncology setting. PCI-27483 inhibits coagulation factor VIIa which is a serine protease. Tissue factor localizes VIIa to the cell membrane and it has been suggested that the tissue factor:VIIa complex promotes pro-angiogenic signals in tumors [7]. Adenovirus mediated HSV gene delivery followed by anti-herpetic drugs has been tested in glioblastoma [15], retinoblastoma [16], hepatocellular carcinoma [17] and head and neck tumors [18] previously. It has been shown both promising and safe in all those settings. Given the results of Bloomston et al. [11] (Abstract #195), it remains to see whether this strategy is of any value in pancreatic cancer phase II studies.
Overall it appears that a number of novel compounds are ready to be tested in phase II trials in patients with pancreatic cancer taking into account their reasonable toxicity spectrum as presented in the 2011 ASCO GI Cancer Symposium.
The authors have no potential conflict of interest