Keywords
Nafion covered lead film glassy carbon electrode; Adsorptive stripping determination; Paracetamol determination; Pharmaceutical analysis
Introduction
Paracetamol (acetaminophen, N-acetyl-p-amino-phenol, PA) is a widely used pain reliever and fever reducer. At normal therapeutic doses, PA is metabolized very fast and fully eliminated in urine [1]. However, overdose of PA can lead to accumulation of toxic metabolites which may cause hepatotoxicity and nephrotoxicity [2]. Because of its broad therapeutic use, it is necessary to develop fast, simple and accurate methodologies for determination of paracetamol for medical control in biological fluids and quality control analysis in pharmaceutical formulations. Therefore, many methods have been developed to determine acetaminophen including titrimetry [3,4], UV/VIS spectrophotometry [5-7], spectrofluorometry [8-11], chromatography [12-15]. However, these methods are burdened with some disadvantages such as high cost, long analysis time and the need for sample preparation.
Electrochemical method has attracted much attention because of quick response, high sensitivity, as well as low-cost analysis. Since paracetamol is an electroactive compound, electrochemical sensors represent an interesting alternative for its quantification. Most electrochemical methods are based on the use of modified carbon - based electrodes such as modified carbon-ceramic electrode with single-walled carbon nanotubes (SWCNT/CCE) [16,17], modified edge plane pyrolytic graphite electrode with single-walled carbon nanotubes (SWCNT/EPPGE) [18], basal plane pyrolytic graphite electrode modified by multi-walled carbon nanotubes (MWCNT-BPPGE) [19], polyaniline-multiwalled carbon nanotubes composite modified electrode (PANI-MWCNT) [20], luteolin/functionalized multi-walled carbon nanotubes modified glassy carbon electrode (Lt/ fMWCNT/MGCE) [21], gold nanoparticles multi-walled carbon nanotube modified glassy carbon electrode (AuNPs/MWCNT/GCE) [22], 8,9-dihydroxy-7-methyL-12Hbenzothiazolo[ 2,3b]quinazolin-12-one modified multi-walled carbon nanotubes paste electrode (DMBQ-MWCNTPE) [23], Cu-poly-terthiophene carboxylic acid modified glassy carbon electrode (Cu-poly-TTCA) [24], carbon–coated nickel magnetic nanoparticles modified glassy carbon electrode (CNi/ GCE) [25], nanoparticles modified glassy carbon electrode (ERG/Ni2O3-NiO/GCE) [26], graphene-modified glassy carbon electrode (Graphene/GCE) [27], graphite and polyurethane screen printed composite electrode (EIGPU) [28], poly(3,4- ethylenedioxythiophene) modified screen printed electrode (PEDOT/SPE) [29], poly(3,4-ethylenedioxythiophene)/ graphene oxide composite modified glassy carbon electrode (PEDOT/GO/GCE) [30], graphene/nickel oxides (Ni2O3-NiO), C60 modified glassy carbon electrode (C60/GCE) [31]. Recently, the boron-doped diamond electrode modified with Nafion and lead films (PbF/Nafion/BDDE) was applied for simultaneous voltammetric determination of paracetamol and ascorbic acid [32]. It should be also noted, that a glassy carbon electrode modified first by lead film and then by Nafion film (Nafion/ PbF/GCE) was used for determination of caffeine in beverage samples and pharmaceutical formulations [33].
In the present work, a simple and fast procedure was applied for fabrication of modified glassy carbon electrode for the determination of paracetamol by adsorptive stripping voltammetry. It is worth mentioning, that the use of a glassy carbon electrode rather than a boron-doped diamond electrode [32] as a support for Nafion and lead films significantly reduced the cost of paracetamol determination. In the present paper, the procedure of electrode preparation involves two steps. In the first one, the lead film was deposited electrochemically onto a glassy carbon support. Next, a drop of Nafion solution was applied onto the lead film electrode surface. In respect to the electrode modification, the key parameters – the concentration of Pb(II) ions and Nafion solution – were optimized. Then, the effects of accumulation time and accumulation potential of paracetamol were studied. Finally, the analytical performance of this sensor (Nafion/PbF/GCE) for determination of paracetamol in commercial pharmaceutical samples was evaluated.
Experimental
Reagents
All chemicals used were analytical grade and were used without further purification. Paracetamol (PA) and Nafion 5% (w/w) were obtained from Sigma-Aldrich, while HNO3 65%, H2SO4 95%, C2H5OH 96% were purchased from POCh. 0.01 mol L-1 solution of Pb(NO3)2 was prepared from reagent obtained from Sigma. 0.1 mol L-1 HNO3 and 0.1 mol L-1 H2SO4 used as supporting electrolytes were prepared by diluting concentrated solutions. The stock solution of PA (0.01 mol L-1) was prepared in deionized water before starting the set of experiments and stored at 4 ºC in the dark until used. Nafion (5% w/w solution) was diluted with ethanol, in order to reach a 1% w/v concentration. All solutions were prepared using ultra-purified water (>18 MΩ cm) supplied by a Milli-Q system (Millipore, UK).
Apparatus
The voltammetric experiments were performed using μAutolab analyser made by Eco Chemie, the Netherlands. The measurements were carried out using a classical three - electrode quartz cell of 10 mL volume. A modified glassy carbon electrode with the diameter of 1 mm was used as the working electrode. The GC electrode was polished daily using 0.3 μm alumina slurry on a Buehler polishing pad. A platinum wire and Ag/AgCl (3 mol L-1 KCl) were used as auxiliary and reference electrodes, respectively. The pH measurements were made using an Elmetron pH meter CI-316.
Preparation of Nafion/PbF/GCE
The Nafion covered lead film electrode was prepared in two steps. In the first one, the lead film was plated onto a glassy carbon support from 0.1 mol L-1 solution of HNO3 containing 7.5 × 10-5 mol L-1 Pb(NO3)2. The potential of the electrode was changed in the following sequence: 0.5 V for 30 s and -1.4 V for 30 s. The first potential was applied to clean the electrode surface before deposition of lead film. At the potential of -1.4 V lead ions were reduced to the metallic state onto a glassy carbon support. During these steps the solution was stirred using a magnetic stirring bar. After the deposition of the lead film, the electrode was rinsed with deionized water and was left to dry in air for 2 min. In the second step, the Nafion film coating was obtained by applying 0.5 μL of the Nafion solution (1% w/v) onto the lead film electrode surface and allowing the system to dry in air for 2 min in a room temperature.
Procedure of paracetamol determinations at Nafion/PbF/GCE
All voltammetric measurements at the Nafion covered lead film electrode were carried out in 0.1 mol L-1 H2SO4 containing variable concentration of paracetamol. At the potential of -1.45 V for 60 s paracetamol was accumulated by adsorption from stirred solution on the electrode. Then after a rest period of 5 s the anodic square wave voltammograms were registered in the range from -1.45 to 1.0 V, with frequency of 200 Hz, amplitude of 50 mV and step height 2 mV. During the stripping step paracetamol was removed from the modified glassy carbon electrode surface. The measurements were carried out in non-deaerated solutions.
Sample preparation
The determinations of PA performer in commercially available formulations: tablets no. 1 containing 750 mg PA per tablet, tablets no. 2 and 3 containing 500 mg PA per tablet. The pharmaceuticals were prepared by the following procedure. Ten tablets were carefully ground to a fine powder, and then the quantity of homogenous powder equivalent to the average weight per tablet was dissolved in an appropriate amount of deionized water. Tablets no. 1 were dissolved in 250 mL of water supplied by a Milli-Q system. Other pharmaceuticals were dissolved in 25 mL of deionized water by sonication for 15 min and then were filtered using a Millipore filter with average the pore diameter of 0.45 μm. A suitable amount of so prepared samples was added to the supporting electrolyte in the voltammetric cell and analysed directly by adsorptive stripping voltammetry.
Results and Discussion
Voltammetric behaviour of PA at Nafion/PbF/ GCE
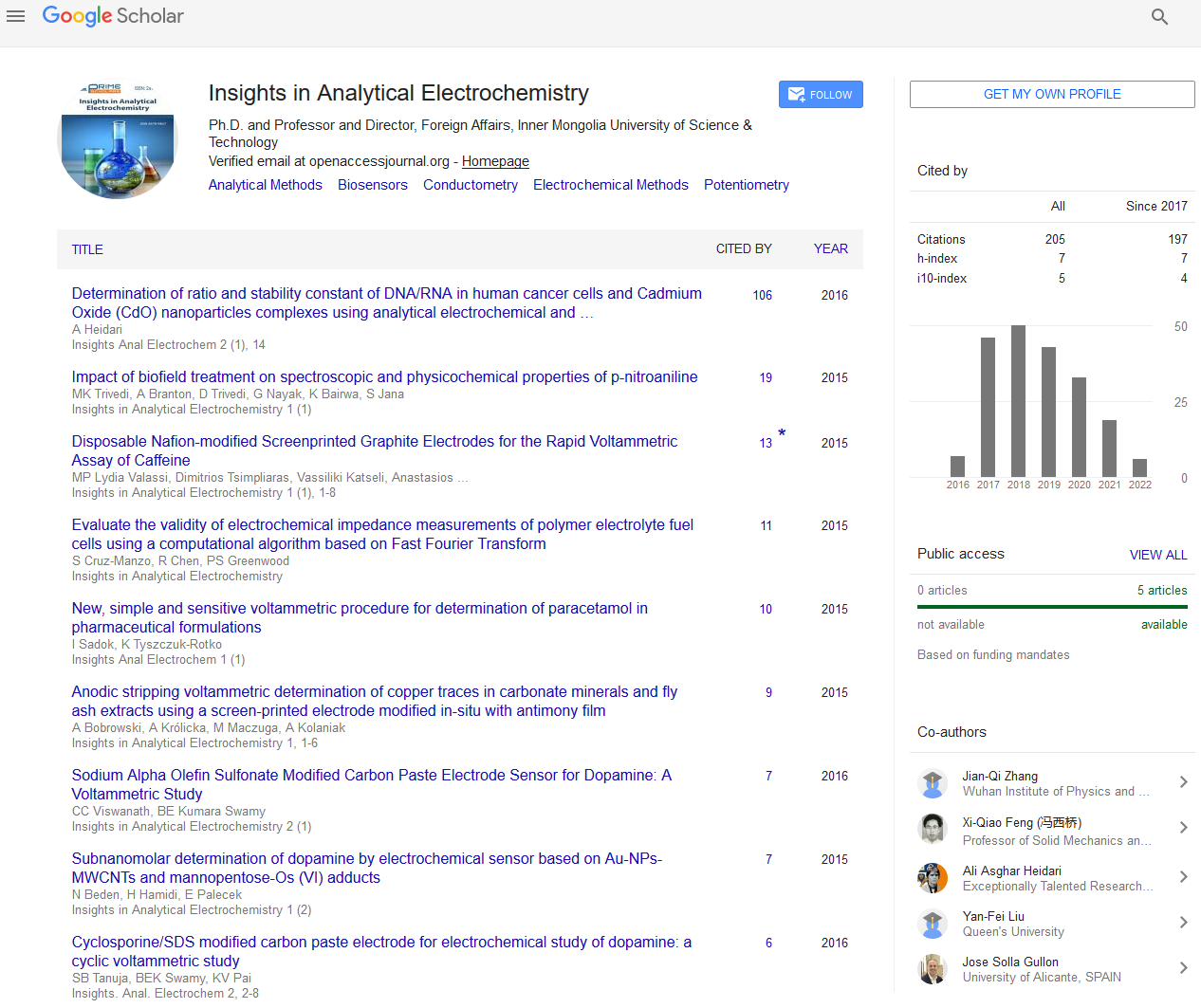
The oxidation mechanism of paracetamol has been investigated in the literature. Oxidation of PA involves a two-electron and twoproton transfer process to form an unstable oxidized product N-acetyl-p-quinoneimine (NAPQI) (Figure 1) [29,31,34].
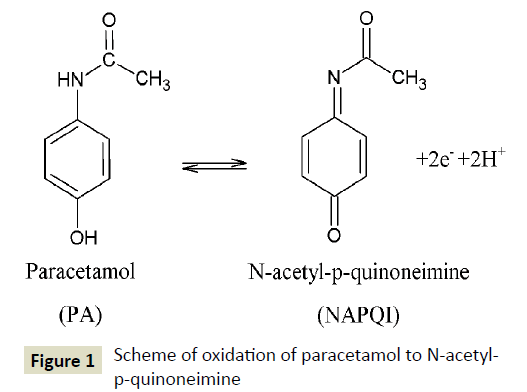
Figure 1: Scheme of oxidation of paracetamol to N-acetylp-quinoneimine
Preliminary electrochemical measurements were carried out in order to evaluate the advantages of the Nafion covered lead film electrode with respect to bare and Nafion covered glassy carbon electrode, ex situ and in situ plated lead film electrode as well as ex situ plated lead film electrode modified with Nafion. Figure 2 shows voltammograms obtained during the determination of paracetamol at these electrodes. In all cases, measurements of paracetamol were carried out in the solution containing 0.1 mol L-1 H2SO4 and 5 × 10-5 mol L-1 paracetamol at the potential of -1.55 V for 120 s. The lead film was plated ex situ from the solution containing 0.1 mol L-1 HNO3 and 7.5 × 10-5 mol L-1 Pb(NO3)2 at the potential of -1.4 V for 30 s. The Nafion film coating was obtained by applying 0.5 μL of the Nafion solution (1% w/v) onto the electrode surface. As it can be seen in Figure 2, the ex situ plated lead film electrode covered with Nafion offers the highest signal of PA and the lowest background, as compared to other electrodes. According to the literature data modification of the surface of the glassy carbon electrode with lead film increases active surface area causing better accumulation of the analyte [35,36]. Furthermore, the advantages of using the Nafion film are related to preconcentration of the analyte in the polymer layer which has already been emphasized by other authors [37,38]. Therefore, the Nafion covered lead film electrode was confirmed as the best choice for further studies.
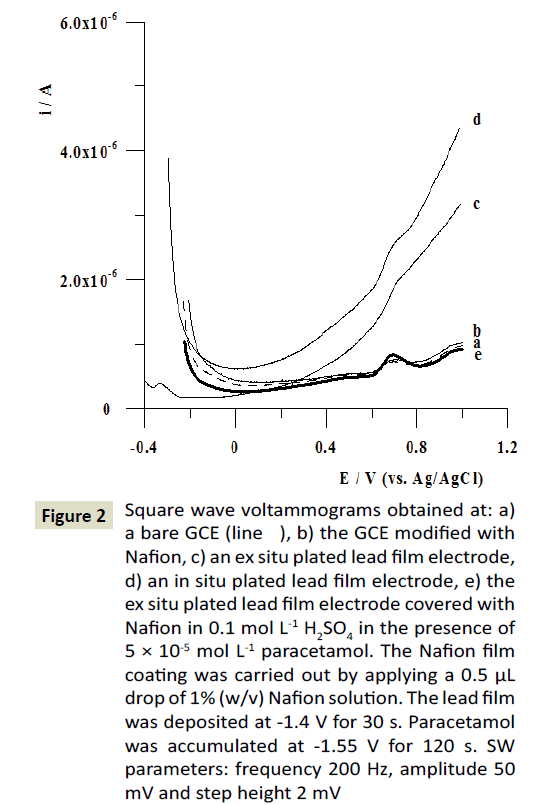
Figure 2: Square wave voltammograms obtained at: a) a bare GCE (line ), b) the GCE modified with Nafion, c) an ex situ plated lead film electrode, d) an in situ plated lead film electrode, e) the ex situ plated lead film electrode covered with Nafion in 0.1 mol L-1 H2SO4 in the presence of 5 × 10-5 mol L-1 paracetamol. The Nafion film coating was carried out by applying a 0.5 μL drop of 1% (w/v) Nafion solution. The lead film was deposited at -1.4 V for 30 s. Paracetamol was accumulated at -1.55 V for 120 s. SW parameters: frequency 200 Hz, amplitude 50 mV and step height 2 mV
Optimization of the electrode modification
In respect to the electrode modification by Nafion and lead films the key parameters, such as the concentration of Pb(II) and Nafion, the volume of Nafion solution applied onto the electrode surface were optimized. The Pb(II) concentration in the lead film plating solution was changed in the range from 0 to 2.5 × 10-4 mol L-1. It was observed that the oxidation peak current of 5 × 10-5 mol L-1 PA attained maximum value when the concentration of Pb(II) was 7.5 × 10-5 mol L-1 (Figure 3A). Next, the effect of the Nafion concentration on the voltammetric response of PA was investigated. The Nafion film coating was carried out by applying a 0.5 μL drop of solution containing Nafion concentration in the range from 0.5 to 2.5% (w/v). The obtained results were shown in Figure 3B. It can be seen that the peak current of PA reached a maximum at 1% w/v Nafion concentration. The results indicate that the Nafion films produced by 0 or 0.5% and higher than 1% w/v Nafion solution were too thin or thick for PA determination. If the Nafion layer is too thin, some parts of the electrode may not be covered and the efficiency may decrease. On the other hand, too thick Nafion film displayed large cracks due to contractive forces within the layer hindering effective mass transport [39]. Then, the volume of Nafion drop applied onto the electrode surface was changed in the range from 0 to 1 μL and influence on the peak current of 5 × 10-5 mol L-1 PA was studied. It was observed that the paracetamol peak attained maximum value for the volume of Nafion drop equal to 0.5 μL. For further experiments a 0.5 μL drop of Nafion solution at concentration of 1% w/v was used for the preparation of the Nafion/PbF/GCE.
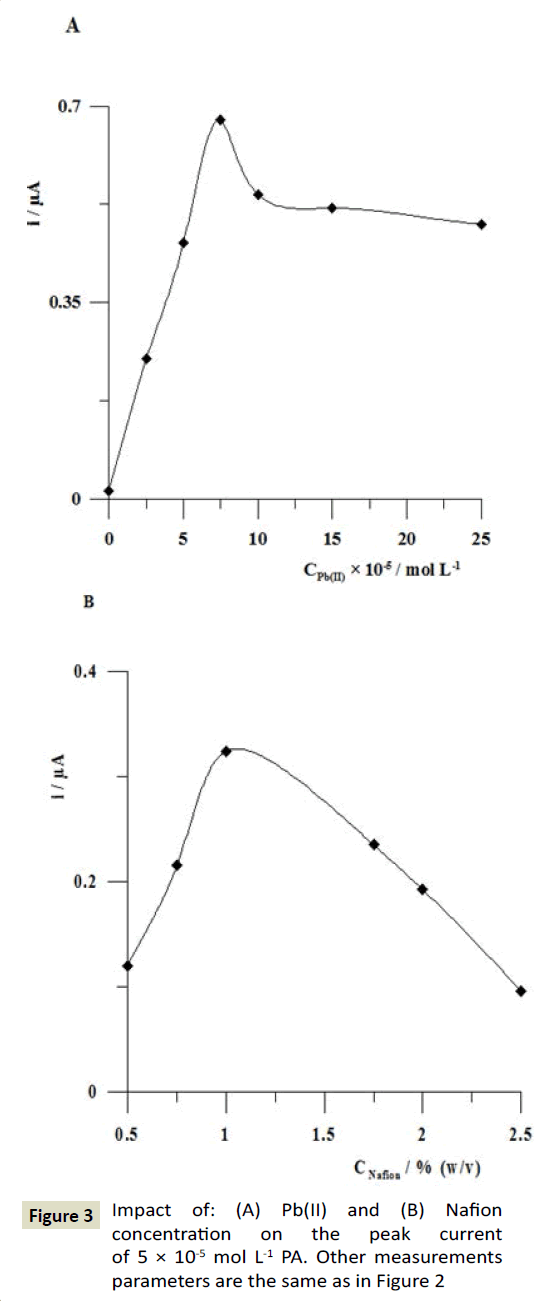
Figure 3: Impact of: (A) Pb(II) and (B) Nafion concentration on the peak current of 5 × 10-5 mol L-1 PA. Other measurements parameters are the same as in Figure 2
Optimization of the paracetamol determination procedure
The influence of accumulation potential on the paracetamol peak current was studied for PA concentration of 5 × 10-5 mol L-1. The potential was changed in the range from -1.4 to -1.6 V. As can be seen in Figure 4A, the oxidation peak of PA attained maximum value at the accumulation potential of -1.45 V, so for further measurements this potential was chosen. The effect of accumulation time was studied for paracetamol concentration of 5 × 10-5 mol L-1. The accumulation time was changed from 0 to 300 s. It was observed that paracetamol response signals increased upon increasing the accumulation time to 60 s and then started to decrease at longer times (Figure 4B). On the basis of these results the accumulation time of 60 s was chosen for further studies.
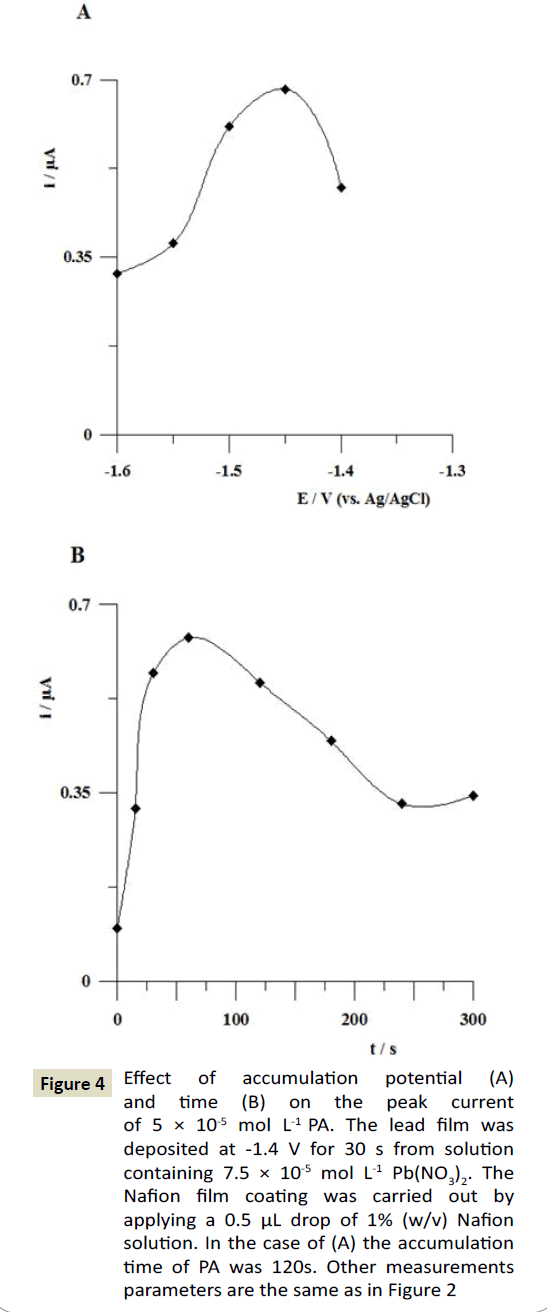
Figure 4: Effect of accumulation potential (A) and time (B) on the peak current of 5 × 10-5 mol L-1 PA. The lead film was deposited at -1.4 V for 30 s from solution containing 7.5 × 10-5 mol L-1 Pb(NO3)2. The Nafion film coating was carried out by applying a 0.5 μL drop of 1% (w/v) Nafion solution. In the case of (A) the accumulation time of PA was 120s. Other measurements parameters are the same as in Figure 2
Calibration graph, repeatability and reproducibility
Under the optimal analytical conditions, determination of paracetamol with increasing concentration was performed. The calibration graph for the accumulation time of 60 s was linear from 5 × 10-7 to 1 × 10-2 mol L-1, and was compliant with the equation y=12.56x+0.297, where y is the peak current (μA) and x is a paracetamol concentration (mmol L-1). The correlation coefficient (R2) was 0.9997. The limits of detection and quantification estimated from 3-times and 5-times the standard deviation (n=5) for the lowest determined concentration of paracetamol were 1.72 × 10-7 mol L-1 and 5.97 × 10-7 mol L-1, respectively.
The repeatability of the signal was determined by successive measurements (n=5) of each studied paracetamol concentration at the same electrode. The relative standard deviation of the peak current in the range from 3.8% to 4.2% was obtained. Moreover, the electrode-toelectrode reproducibility was estimated with five replicate determinations of paracetamol at concentration of 1 × 10-7 mol L-1 and 5 × 10-5 mol L-1 at three independently prepared Nafion/PbF/GC electrodes. The relative standard deviations of the peak current of 5.3% to 4.9% were obtained.
Table 1 shows the comparison of the analytical parameters obtained by the proposed method with those obtained by other electrochemical methods proposed in the literature for the determination of paracetamol. The comparison to other electrochemical sensors for the determination of PA shows that the proposed method using the Nafion covered lead film glassy carbon electrode provides the widest linear range. The limits of detection of PA are lower, comparable or in some cases higher than those obtained using other electrochemical sensors. However, the proposed procedure of the sensor preparation is much simpler than procedures described in the literature with a lower detection limit [19,22,26,27,31]. It has to be noted, that the detection limit obtained at the lead film glassy carbon electrode covered with Nafion film is comparable to that attained using the boron-doped diamond electrode modified with Nafion and lead films [32], but the GCE used as a support in this work is cheaper and easier to buy than the BDDE. In addition, the developed voltammetric procedure using the Nafion/PbF/ GCE offers about two orders of magnitude wider linear range of paracetamol concentration than that obtained at PbF/ Nafion/BDDE [32]. This is most likely related to the microscopic structure of the electrode surface, probably the Nafion/PbF/GCE characterized by a more developed surface. It will be the subject of further research.
| Electrode |
Linear range[μmol L-1] |
Detection limits[μmol L-1] |
Reference |
| SWCNT/CCE |
0.2-150 |
0.12 |
[16] |
| SWCNT/EPPGE |
500-1000000 |
0.029 |
[18] |
| MWCNT-BPPGE |
0.01-2 |
0.01 |
[19] |
| PANI-MWCNT |
1-100 |
25.0 |
[20] |
| Lt/fMWCNT/MGCE |
0.9-80 |
0.78 |
[21] |
| AuNPs/MWCNT/GCE |
0.09-35 |
0.03 |
[22] |
| DMBQ-MWCNTPE |
5-500 |
1.0 |
[23] |
| Cu-poly-TTCA |
20-5000 |
5.0 |
[24] |
| C-Ni/GCE |
7.8-110 |
2.3 |
[25] |
| ERG/Ni2O3-NiO/GCE |
0.04-100 |
0.02 |
[26] |
| Graphene/GCE |
0.1-20 |
0.032 |
[27] |
| EIGPU |
1-40 |
0.84 |
[28] |
| PEDOT/SPE |
4-400 |
1.39 |
[29] |
| PEDOT/GO/GCE |
10-60 |
0.57 |
[30] |
| C60/GCE |
50-1500 |
50 |
[31] |
| PbF/Nafion/BDDE |
0.5-200 |
0.17 |
[32] |
| Nafion/PbF/GCE |
0.5-10000 |
0.172 |
This work |
Table 1: Comparison of various electroanalytical procedures for the determination of paracetamol at modified carbon based electrodes.
Interferences
To investigate the interference effects of some compounds, a fixed amount of 1 × 10-5 mol L-1 paracetamol spiked with various foreign species, such as glucose, fructose, saccharin, sodium carbonate, citric acid, ascorbic acid and acetylsalicylic acid was evaluated under the same experimental conditions. The results showed that at a 10-fold excess these species had not impact on the peaks current of paracetamol (signals changed below 5%).
Real sample analysis
The optimized voltammetric procedure was used with the standard addition method to the determination of paracetamol in commercially available pharmaceutical formulations. The obtained results are shown in Table 2. As can be seen in this table, no significant differences were observed between the label (data supplied by the manufacturer) and found (data obtained by the proposed voltammetric procedure) values of paracetamol in samples. On the basis of obtained results, it can be stated that the proposed method was found suitable for determination of PA in its pharmaceutical formulations without any interference from sample matrices. The voltammograms obtained in the course of PA determination in pharmaceutical formulation no. 2 at the Nafion covered lead film glassy carbon electrode are presented in Figure 5.
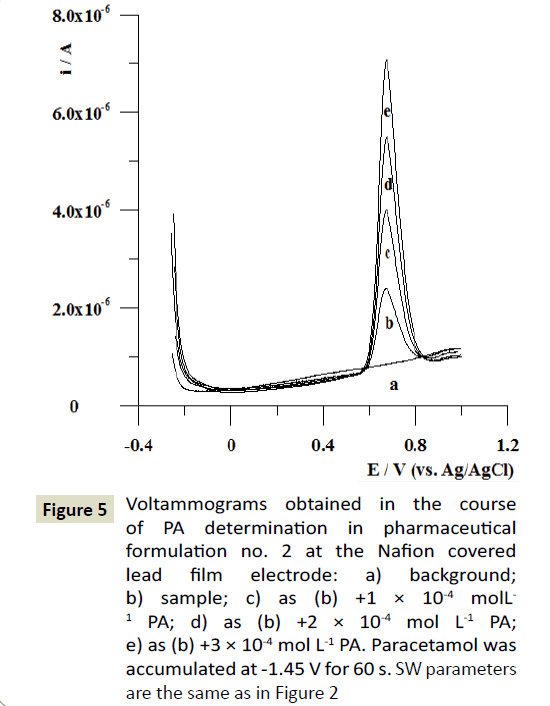
Figure 5: Voltammograms obtained in the course of PA determination in pharmaceutical formulation no. 2 at the Nafion covered lead film electrode: a) background; b) sample; c) as (b) +1 × 10-4 molL-1 PA; d) as (b) +2 × 10-4 mol L-1 PA; e) as (b) +3 × 10-4 mol L-1 PA. Paracetamol was accumulated at -1.45 V for 60 s. SW parameters are the same as in Figure 2
| Pharmaceuticalformulation |
Label value(mg/tablet) |
Found value ± RSD(mg/tablet) ± (%) |
Relative error(%) |
| 1 |
750 |
740.9 ± 3.3 |
-1,2 |
| 2 |
500 |
493.5 ± 3.0 |
-1,3 |
| 3 |
500 |
502.0 ± 3.3 |
0.4 |
Table 2: The results of PA determination in pharmaceutical formulations (no. 1, 2, 3) obtained by the proposed voltammetric procedure.The relative standard deviations (RSD) are given in % for n=5. The relative error (%)=100 × (found value-label value)/label value.
Conclusions
The approach adopted in this work provides a new voltammetric procedure for determination of paracetamol using a Nafion covered lead film glassy carbon electrode in pharmaceutical formulation. The proposed procedure is characterized by major advantages, such as short determination time, low cost, very wide linear range of paracetamol concentrations, low detection and quantification limits and no impact of the pharmaceutical sample matrix on PA signals. Thanks to these properties, the developed procedure using the Nafion/PbF/GCE provides a useful tool for the detection and quantification of paracetamol in commercial formulation.
References
- Espinosa Bosch M, Ruiz Sánchez AJ, Sánchez Rojas F, Bosch Ojeda C (2006) Determination of paracetamol: historical evolution. J Pharm Biomed Anal 42: 291-321.
- Martin FL, McLean AE (1998) Comparison of paracetamol-induced hepatotoxicity in the rat in vivo with progression of cell injury in vitro in rat liver slices. Drug ChemToxicol 21: 477-494.
- Kumar KG, Letha R (1997) Determination of paracetamol in pure form and in dosage forms using N,N-dibromodimethylhydantoin. J Pharm Biomed Anal 15: 1725-1728.
- Burgot C, Auffret F, Burgot JL (1997) Determination of acetaminophen by thermometric titrimetry. Anal ChimActa 343: 125-128.
- Dunkerley S, Adams MJ (1997) The simultaneous determination of caffeine, aspirin and paracetamol by principal components regression using automatic dilution and calibration. Lab AutomInf Manage 33: 107-117.
- Nagaraja P, Murthy KC, Rangappa KS (1998) Spectrophotometric method for the determination of paracetamol and phenacetin. J Pharm Biomed Anal 17: 501-506.
- Rodenas V, García MS, Sánchez-Pedreño C, Albero MI (2000) Simultaneous determination of propacetamol and paracetamol by derivative spectrophotometry. Talanta 52: 517-523.
- Nakamura H, Tamura Z (1980) Fluorimetric determination of secondary amines based on their reaction with fluorescamine. Anal Chem 52: 2087-2092.
- Shibasaki J, Konishi R, Yamada K (1980) Fluorometric determination of acetaminophen and its conjugates in whole blood. Chem Pharm Bull (Tokyo) 28: 669-672.
- Shibasaki J, Konishi R, Yamada K, Matsuda S (1982) Improved fluorometric determination of acetaminophen and its conjugates with 1-nitroso-2-naphthol in whole blood and urine. Chem Pharm Bull (Tokyo) 30: 358-361.
- Vilchez JL, Blanc R, Avidad R, Navalón A (1995) Spectrofluorimetric determination of paracetamol in pharmaceuticals and biological fluids. J Pharm Biomed Anal 13: 1119-1125.
- Di PietraAM, Gatti R, Andrisano V, Cavrini V (1996) Application of high-performance liquid chromatography with diode-array detection and on-line post-column photochemical derivatization to the determination of analgesics. J Chromatogr A 729: 355-361.
- Haque A, Stewart JT (1999) Determination of acetaminophen, caffeine, and butalbital in a commercial tablet dosage form by micellarelectrokinetic chromatography. J LiqChromRelat Tech 22: 2159-2166.
- Pérez JL, Bello MA (1999) Determination of paracetamol in dosage forms by non-suppressed ion chromatography. Talanta 48: 1199-1202.
- Speed DJ, Dickson SJ, Cairns ER, Kim ND (2001) Analysis of paracetamol using solid-phase extraction, deuterated internal standards, and gas chromatography-mass spectrometry. J Anal Toxicol 25: 198-202.
- Habibi B, Jahanbakhshi M, Pournaghi-Azar MH (2011) Differential pulse voltammetric simultaneous determination of acetaminophen and ascorbic acid using single-walled carbon nanotube-modified carbon-ceramic electrode. Anal Biochem 411: 167-175.
- Habibi B, Jahanbakhshi M, Pournaghi-Azar MH (2011) Simultaneous determination of acetaminophen and dopamine using SWCNT modified carbon–ceramic electrode by differential pulse voltammetry. ElectrochimActa 56: 2888-2894.
- Goyal RN, Gupta VK, Chatterjee S (2010) Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sensor Actuat B-Chem 149: 252-258.
- Kachoosangi RT, Wildgoose GG, Compton RG (2008) Sensitive adsorptive stripping voltammetric determination of paracetamol at multiwalled carbon nanotube modified basal plane pyrolytic graphite electrode. Anal ChimActa 618: 54-60.
- Li M, Jing L (2007) Electrochemical behaviour of acetaminophen and its detection on the PANI–MWCNTs composite modified electrode. ElectrochimActa 52: 3250-3257.
- Amiri-Aref M, Raoof JB, Ojani R (2014) A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sensor Actuat B-Chem 192: 634-641.
- Madrakian T, Haghshenas E, Afkhami A (2014) Simultaneous determination of tyrosine, acetaminophen and ascorbic acid using gold nanoparticles/multiwalled carbon nanotube/glassy carbon electrode by differential pulse voltammetric method. Sensor Actuat B-Chem 193: 451-460.
- Karimi-Maleh H, Moazampour M, Ahmar H, Beitollahi H, Ensafi AA, et al. (2014) A sensitive nanocomposite-based electrochemical sensor for voltammetric simultaneous determination of isoproterenol, acetaminophen and tryptophan. Measurement 51: 91-99.
- Boopathi M, Won MS, Shim YB (2004) A sensor for acetaminophen in a blood medium using a Cu(II)-conducting polymer complex modified electrode. Anal ChimActa 512: 191-197.
- Wang SF, Xie F, Hu RF (2007) Carbon-coated nickel magnetic nanoparticles modified electrodes as a sensor for determination of acetaminophen. Sensor Actuat B-Chem 123: 495-500.
- Liu GT, Chen HF, Lin GM, Ye PP, Wang XP, et al. (2014) One-step electrodeposition of graphene loaded nickel oxides nanoparticles for acetaminophen detection. BiosensBioelectron 56: 26-32.
- Kang X, Wang J, Wu H, Liu J, Aksay IA, et al. (2010) A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 81: 754-759.
- Saciloto TR, Cervini P, Cavalheiro ÉTG (2013) Simultaneous voltammetric determination of acetaminophen and caffeine at a graphite and polyurethane screen-printed composite electrode. J BrazChemSoc 24: 1461-1468.
- Wang-Yu S, Shu-Hua C (2010) Electrochemical oxidation and sensitive determination of acetaminophen in pharmaceuticals at poly(3,4-ethylenedioxythiophene)-modified screen-printed electrodes. Electroanal 22: 707-714.
- Si W, Lei W, Han Z, Zhang Y, Hao Q, et al. (2014) Electrochemical sensing of acetaminophen based on poly(3,4-ethylenedioxythiophene)/graphene oxide composites. Sensor Actuat B-Chem 193: 823-829.
- Goyal RN, Singh SP (2006) Voltammetric determination of paracetamol at C-60- modified glassy carbon electrode. ElectrochimActa 51: 3008-3012.
- Tyszczuk-Rotko K, B?czkowska I, Wójciak-Kosior M, Sowa I (2014) Simultaneous voltammetric determination of paracetamol and ascorbic acid using a boron-doped diamond electrode modified with Nafion and lead films. Talanta 129: 384-391.
- Tyszczuk-Rotko K, B?czkowska I (2015) Nafion covered lead film electrode for the voltammetric determination of caffeine in beverage samples and pharmaceutical formulations. Food Chem 172: 24-29.
- Nematollahi D, Shayani-Jam H, Alimoradi M, Niroomand S (2009) Electrochemical oxidation of acetaminophen in aqueous solutions: Kinetic evaluation of hydrolysis, hydroxylation and dimerization processes. ElectrochimActa 54: 7407-7415.
- Bobrowski A, Kalcher K, Kurkowska K (2009) Microscopic and electrochemical characterization of lead film electrode applied in adsorptive stripping analysis. ElectrochimActa 54: 7214-7221.
- Tyszczuk-Rotko K (2012) Influence of Pb(II) concentration and pH of acetate buffer on the potential window of a lead film electrode: an Atomic Force Microscopy Study. MicroscMicroanal 18: 531-537.
- Brunetti B, Desimoni E, Casati P (2007) Determination of caffeine at a Nafion-covered glassy carbon electrode. Electroanal 19: 385-388.
- Martínez-Huitle CA, SuelyFernandes N, Ferro S, De Battisti A, Quiroz MA, et al. (2010) Fabrication and application of Nafion®-modified boron-doped diamond electrode as sensor for detecting caffeine. Diamond Relat Mater 19: 1188-1193.
- Kefala G, Economou A, Voulgaropoulos A (2004) A study of Nafion-coated bismuth-film electrodes for the determination of trace metals by anodic stripping voltammetry. Analyst 129: 1082-1090.