Review Article - (2018) Volume 0, Issue 0
Department of Diagnostic Pathology, Jichi Medical University Hospital, Tochigi, Japan
Received Date: October 11th, 2017; Accepted Date: November 15th, 2017
Neuroendocrine neoplasms of the pancreas are relatively rare, accounting for approximately 1–2% of all pancreatic neoplasms, and are composed of epithelial neoplastic cells with neuroendocrine differentiation. Neuroendocrine neoplasms are potentially malignant neoplasms including well-differentiated types (neuroendocrine tumors, neuroendocrine tumors) and poorly differentiated types (neuroendocrine carcinomas). The WHO classification released in 2010 led to a significant change in the grading system of neuroendocrine neoplasms of the digestive system. “Endocrine neoplasm” was changed to “neuroendocrine neoplasm”. Neuroendocrine neoplasms are graded according to the number of mitoses and/or Ki-67 index. These changes simplified the classification scheme. However, there are a number of remaining issues. Neuroendocrine tumors meeting the WHO criteria for neuroendocrine carcinoma (>20 mitoses/10 high power fields and/or Ki67 index > 20%) with a well-differentiated morphology, known as an “organoid pattern” have been identified. In the revised version of the “WHO Classification of Tumours of Endocrine Organs” published in 2017, to solve the problems of high-grade (grade 3) neuroendocrine neoplasms, they are divided into pancreatic neuroendocrine tumors, grade 3 (PanNET G3) and neuroendocrine carcinomas, grade 3 (PanNEC G3) depending on their histo-morphologic characteristics. The neuroendocrine tumor G3 category is associated with a better prognosis and does not significantly responds to cisplatin-based chemotherapy. Defining that subgroup of patients using a combination of tumor morphology and cell proliferation is important. Better strategies to treat and improve the outcomes of patients with pancreatic neuroendocrine neoplasms are required.
Carcinoma; Classification; Neuroendocrine Tumors; Pancreas
NEC neuroendocrine carcinoma; NENs neuroendocrine neoplasms; NET neuroendocrine tumors
Neuroendocrine neoplasms (NENs) of the pancreas are composed of epithelial neoplastic cells with phenotypic neuroendocrine differentiation. NENs are potentially malignant tumors including well-differentiated types (neuroendocrine tumors, NETs) and poorly differentiated types (neuroendocrine carcinomas, NECs) [1]. These tumors are relatively rare, but are the second most common neoplasm of the pancreas, accounting for approximately 1–2% of all pancreatic neoplasms [1, 2]. The incidence of NETs of the pancreas has increased recently [3, 4].
In this short review updated clinicopathological features and grading system of the pancreatic NEN are described.
Patients’ Characteristics and Clinical Diagnosis
Patients with NETs are typically from 30 to 60 years old, and show no significant gender predilection [1, 2, 3, 4]. Because of lacking specific symptoms, non-functional pancreatic NENs tend to be diagnosed at more advanced stages of disease compared with functional pancreatic NENs such as insulinoma and gastrinoma. Approximately 1–2% of patients with these tumors have predisposing familial syndromes. However, patients with NEC tend to be older, similar to patients with pancreatic ductal adenocarcinoma.
In addition to a physical examination, imaging studies including US, CT and MRI are useful to diagnose as NEN. In addition, the endoscopic ultrasonography-guided fineneedle aspiration (EUS-FNA) biopsy has enabled correct pathological diagnosis and suitable treatment for the tumors.
The WHO tumor classification of endocrine organs [5] published in 2004 divided NENs into well-differentiated NETs and well- and poorly differentiated neuroendocrine carcinoma (NEC), and the former NETs were sub-classified into benign and borderline tumors according to tumor size, mitotic rate, lymphovascular invasion and perineural invasion [5]. The 2010 WHO classification included several major changes in the grading system of neuroendocrine neoplasms of the digestive system. “Endocrine neoplasm” was changed to “neuroendocrine neoplasm”, NENs are now graded according to the number of mitoses and/or Ki-67 index (using the MIB1 antibody), and this grading system is applied to NENs arising in any organ of the digestive system [1]. These changes were based on the Europian Neuroendocrine Tumor Society (ENETS) consensus guideline [6]. In this classification, NENs were divided into well-differentiated NETs (NET G1 and G2) and poorly differentiated NEC. NET G1 was defined as having a Ki 67 index of ≤2% and <2 mitoses/10 high power fields (HPF). NET G2 was defined as a Ki-67 index of 3 to 20% or 2 to 20 mitoses/10HPFs. NEC was defined as a Ki-67 index of >20% and >20 mitoses/10HPFs. The WHO recommends that for mitotic counts, at least 50 HPFs should be counted, and for Ki-67 index, a minimum of 500 tumor cells should be counted in tumor hot spots. For grade-discordant cases (based on differences in mitotic count and Ki-67 index), the higher grade should be used.
These changes simplified the classification scheme, however several issues remain. NECs of the pancreas are defined by cell proliferation criteria only, however NENs meeting the WHO criteria for NEC (described above) with a well-differentiated morphology and an “organoid pattern” have been identified [7, 8]. Other lesions have components of a well differentiated NET with a low proliferative rate, and are admixed with a high-grade NEN within the specimen from the same patient. These cases have been interpreted as high-grade progression of a well differentiated NEN [9]. Heterogeneity within the neoplasm should be noted, especially in FNA samples. The revised version of the “WHO Classification of Tumours of Endocrine Organs”, published in 2017, divided high grade NENs into neuroendocrine tumor, Grade 3 (PanNET G3) and neuroendocrine carcinomas, Grade 3 (PanNEC G3) according to their histo-morphologic characteristics (Table 1) [10]. Tumors in the NET G3 category are associated with a better prognosis and do not significantly respond to cisplatin-based chemotherapy. Rb and KRAS are promising predictors of response to platinum-based chemotherapy for NEN G3 tumors, and Rb for PanNEC (G3) [8].
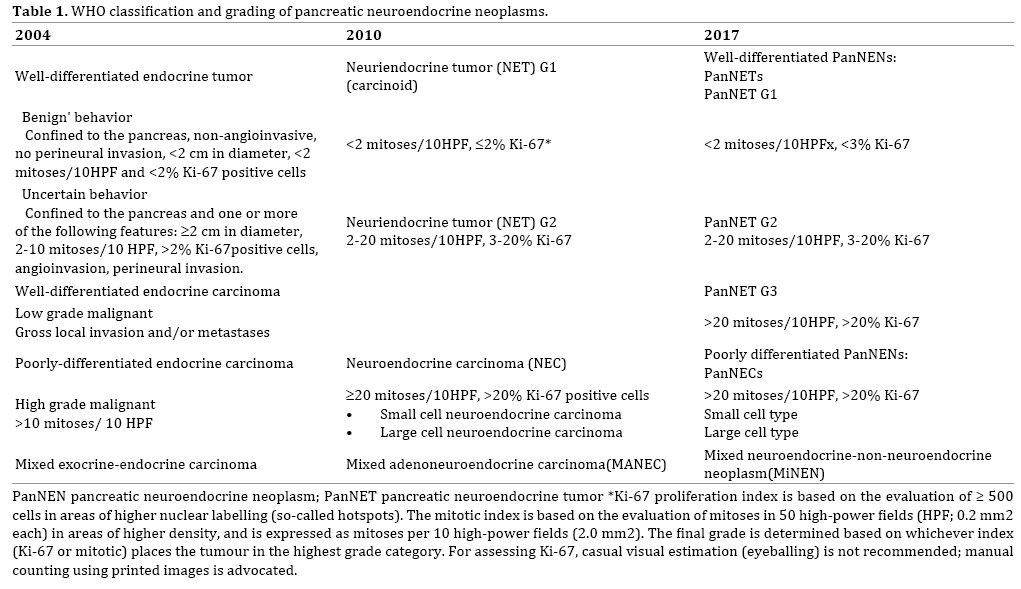
The Japanese classification of pancreatic cancer, 7th edition, was released by Japan Pancreas Society (JPS) in July 2016 and English version of it was in 2017 [11]. In this book, 2010 WHO grading system of NENs was adopted and concerning to so-called “NET G3-issue” they commented on it as “… some NETs with organoid structures may also demonstrate a high proliferative potential (Ki67 index >20%, mitotic count >20 per HPF). These are sometimes called well differentiated NECs or NET G3.”
ENETS first proposed a staging system for NETs, and this was accepted in the 7th edition of the American Joint Committee on Cancer (AJCC)/ Union for International Cancer Control (UICC) TNM staging manual [12]. Recently revised AJCC/UICC system divided it each organ such as stomach, duodenum/ampullary, jejunum/ileum, appendix, colon and rectum [13].
Unlike in the case of digestive organ, the ENETS recommendations and WHO 2015 classifications of lung and thymus NENs take into account the grade of necrosis in addition to Ki-67, and define three distinct subgroups: typical carcinoid, atypical carcinoid (that would correspond to NET G1 and G2, respectively) and large- and small-cell NECs [14].
Gross Findings
Grossly, NETs (well-differentiated NENs) are usually solitary, well circumscribed, tan to pink, and relatively homogeneous soft tumors, however they may be hard (sclerotic) with gray-white nodules (Figure 1a), yellowish nodules (Figure 1b) or cystic tumors. NECs (poorly differentiated NENs) are usually tan-red or yellowish, solid masses and frequently have hemorrhagic and/or necrotic areas.
Well-Differentiated and Low Grade NENs (Nets G1 and G2)
Well-differentiated NENs usually have an organoid architecture including solid nests, trabeculae, ribbon-like, glandular, acinar, and rosette formations, lacking necrosis, and are composed of monotonous epithelial cells with a fair amount of cytoplasm and regular round nuclei with a so-called “salt and pepper” appearance (Figures 2a and 2b). Most tumors have rich vascular networks. NETs in this category have a Ki-67 index of ≤20% and ≤20 mitoses/10 HPF (Figure 2c). Vascular invasion is often found, even in these low grade tumors (Figure 2d).
Figure 2: Histologic features of pancreatic neuroendocrine tumors (NETs). (a). The tumor has a so-called “organoid structure” including ribbonlike and pseudo- rosette patterns. (b). The tumor has a so-called “organoid structure” including trabeculae with a thin vascular network. (c). Ki-67 immunohistochemistry (WHO NET G2). (d). Vascular invasion.
Several functioning NETs show characteristic histologic findings [2]. Stromal amyloid deposition is frequently seen in insulinomas. Glandular formation with psammomatous calcification is sometimes noted in somatostatin-producing tumors (Figure 3a). Approximately 10% of NETs are accompanied with dense stromal fibrosis (Figure 3b). These NETs are often serotonin positive and sometimes show duct involvement.
There are also many histo-morphologic variants including clear cell/lipid rich, oncocytic, pleomorphic, rhabdoid, glandular, and others. NETs with morphologically abundant clear cytoplasm (“lipid-rich”) (Figure 3c), grossly mimic adrenal cortical neoplasms, and are seen especially in patients with VHL syndrome [15], but sporadic cases have also been reported [16]. “Pigmented black” pancreatic neuroendocrine tumors are composed of intracytoplasmic lipofuscin and mimic metastatic melanoma [17]. Oncocytic NETs are characterized by cells with abundant granular eosinophilic cytoplasm because of accumulation of mitochondria (Figure 3d) [18]. Some NETs show marked nuclear atypia/pleomorphism throughout the tumor. Although these NETs have the possibility of being undifferentiated carcinomas or poorly differentiated ductal carcinomas, no elevated mitotic rate or aggressive biology is seen [19]. Some NETs have abundant ductal components with obvious benign cytology [2, 19]. It is sometimes debatable whether those ductules are neoplastic glandular components of NETs or entrapped proliferating non-neoplastic ductules. If the ductular components have apparent histologic atypia, mixed ductal-neuroendocrine carcinoma should be considered. However these are exceedingly uncommon [19].
Well-Differentiated and High Grade NENs, (NET G3)
Pancreatic NET G3 lesions are defined by a Ki- 67 proliferation index >20% and/or a mitotic index >20/10HPF and also have organoid structures as described above (Figures 4a and 4b) [10]. The upper limit for the proliferation index has not been defined, however the Ki-67 index is usually less than 55%. Only low immunohistochemical expression of p53 or loss of Rb is observed. Due to their morphological similarity, it is necessary to differentiate them from acinar cell carcinomas by immunohistochemistry (Figures 4c and 4d).
Figure 4: Histologic features of pancreatic neuroendocrine tumors (NETs)(WHO PanNET G3). (a). The tumor has a glandular or acinar-like structure. (b). Ki-67 immunohistochemistry (>20%/HPF). (c). Tumor cells are partially positive for chromogranin A. (d). Tumor cells are diffusely positive for synaptophysin.
Poorly Differentiated and High Grade NENs (NEC G3)
Poorly differentiated NECs (NEC G3) are defined by a Ki-67 proliferation index >20% and/or a mitotic index >20/10HPF with no differentiated morphology (organoid structures) [10]. NECs G3 are divided into small cell NEC and large cell NEC. Small cell NEC of the pancreas share a similar morphology with small cell carcinoma of the lung, and it is important to rule out metastases to the pancreas before establishing the diagnosis. NEC G3 tumors typically have sheets or nests of carcinoma cells with pleomorphic, hyperchromatic nuclei and abundant mitotic figures (Figure 5). The so-called "salt and pepper" chromatin pattern is lost. Necrosis is often present in these solid nests. Mixed tumors with exocrine differentiation are aggressive lesions that behave more like exocrine than endocrine carcinomas.
Diagnostic Markers
Well differentiated-NENs are defined by the existence of cytoplasmic neuroendocrine granules, and the currently accepted reliable markers are synaptophysin and chromogranin A. Well-differentiated NENs tend to show stronger and more diffuse staining with these neuroendocrine markers than poorly differentiated NENs. CD56 antibody, against neural cell adhesion molecules, is less specific as a neuroendocrine marker.
Approximately 45% of sporadic well differentiated- NENs show loss of expression of DAXX and ATRX immunohistochemically, which correlates with mutations in the DAXX and ATRX genes [20, 21]. Immunohistochemically detected peptides do not imply that the patient has clinical symptoms, and the opposite situation may occur, probably due to the rapid release and dispersal of the hormone product without intracytoplasmic accumulation [2].
Prognostic or Therapy Related Markers
The mitotic count and the Ki-67/MIB-1-labeling index are the most reliable prognostic markers for NENs. Cytokeratin 19 (CK 19) is usually regarded as a marker of ductal epithelial cells and is expressed not only by poorly differentiated but also by well-differentiated NENs. Several studies reported that CK 19 is a marker of more aggressive behavior [22, 23, 24]. c-Kit has also reported as a worse prognostic marker [22, 24]. There are several other prognostic markers which were previously reported such as CD99, CD44, p27, epidermal growth factor (EGF), hepatocyte growth factor receptors (HGFR), E-cadherin, CEACAM1, HER-2 and c-MET. However, they have not been validated in clinical use [22, 25, 26, 27, 28].
Somatostatin, which binds to a family of five G-proteincoupled receptors, was identified as an important inhibitory hormone. The expression of somatostatin receptors (SSTRs) in NETs, as shown by octreotide scintigraphy and immunocytochemistry, led to the application of this inhibitor in the treatment of patients with NETs [29]. The development of long-acting somatostatin analogs allowed for clinical use because the native somatostatin has a very short half-life of only 2 minutes. SSTR2 is expressed by most pancreatic NETs and shows high affinity for somatostatin analogs. It has been used as a target for molecular imaging and treatment of NETs [29]. Immunohistochemistry for SSTR2A is widely performed.
The histologic differential diagnosis of pancreatic NETs includes pancreatic neoplasms showing solid or diffuse cellular proliferation as well as metastatic neoplasms. Furthermore, it is not always easy to distinguish a NET G3 from NEC G3 tumors by morphology only. Tang et al. proposed a diagnostic algorithm for high-grade NENs using a combination of histology and immunohistochemistry for7 DAXX/ATRX, Rb and p53 [5].
In primary pancreatic neoplasms, acinar cell carcinoma (ACC) should first be ruled out because their radiologic/ macroscopic and microscopic morphology resembles NETs. It is practically difficult to differentiate between a high-grade NET (NET G3) and ACC only by morphology. A panel of immunohistochemical stains including both neuroendocrine and acinar cell markers (trypsin and BCL10) can help to distinguish these lesions.
Solid pseudopapillary neoplasms (SPNs), showing solid sheet-like, nest and sometimes a rosette-like appearance, also resemble NETs. Immunohistochemical nuclear staining of beta-catenin is very useful to diagnose it as a SPN. Distinguishing from a mixed acinar-neuroendocrine carcinoma may be challenging.
Metastatic clear cell carcinomas, known as one of the most frequent tumors to metastasize only to the pancreas, resemble the clear cell or lipid-rich variant of NET. The oncocytic variant of NET may resemble hepatocellular carcinoma.
Prognosis
Surgical complete resection of the tumor is an only curative treatment for low grade NETs (NET G1 or G2). If untreated, most pancreatic NETs grow and eventually metastasize to the liver. Recently, several treatment options in the setting of metastatic disease have been developed, which include systemic treatment with somatostatin analogs (SSAs), interferon-α (INF-α), peptide receptor radiotargeted therapy (PRRT), cytotoxic chemotherapy or molecular target agents such as everolimus and sunitinib [30, 31].
Most PNETs are indolent but have malignant potential. Overall 10-year survival of the patients with low grade NENs (NET G1 or G2) is 60-70%, and with both low grade (G1) and low stage (T1N0M0) is more than 95% [19]. Overall 5-year survival of the patients with NET G2, G3 and NEC G3 is 61%, 22%, and 17%, respectively [19].
The WHO classification published in 2010 had a significant impact on the classification and grading of pancreatic NENs. However, a number of issues remain. The revised version of the WHO classification published in 2017 proposed grading criteria based on both cell proliferation and morphology. Better strategies, depending on appropriate pathological evaluation, to treat and improve the outcomes of the patients with pancreatic NENs are required.
We thank Professor Alan Lefor, The Center for Graduate Medical Education, Jichi Medical University, for comments that greatly improved the manuscript.
All authors declare having no conflict of interests.