Gatto L1,2, Ramazzotti V1, Micari A3 and Prati F1,2*
1Interventional Cardiology, San Giovanni Addolorata Hospital, Rome, Italy
2Centro per la Lotta Contro L’Infarto - CLI Foundation, Rome, Italy
3GVM Care and Research, E. S. Health Science Foundation, Cotignola, Italy
*Corresponding Author:
Prati F
Interventional Cardiology, San Giovanni
Addolorata Hospital, Via dell’Amba Aradam
8, 00184, Rome – Italy
Tel: +39 06 77.05.53.30
Fax: +39 06 77.05.53.30
E-mail: fprati@hsangiovanni.roma.it
Received date: January 17, 2017; Accepted date: May 11, 2017; Published date: May 18, 2017
Citation: Gatto L, Ramazzotti V, Micari A, et al. Mechanical Causes of Stent Failure: OCT Insights. Interv Cardiol J 2017, 3:2. doi:10.21767/2471-8157.100054
Introduction
Despite a constant evolution in stent/scaffold design, stent failure is still perceived as a clinical problem. The process of drug eluting stent (DES) struts thinning that occurred in the last year with the introduction of cobalt-chromium devices has been of utmost importance to significantly reduce the incidence of stent thrombosis and restenosis [1,2]. Yet, suboptimal stent positioning represents a trigger for acute events even after deployment of scaffolds with optimal design and a valid polymerdrug combination [3-7].
The aim of this review is to highlight the role of Optical Coherence Tomography (OCT) in guiding PCI (Percutaneous Coronary Intervention) to prevent some features of suboptimal stent implantation related with higher risk of cardiac events during the follow-up.
Findings
The CLI-THRO study addressed the incidence of suboptimal OCT results in 21 consecutive patients exhibiting subacute thrombosis [8]. The patients were matched 1:2 with a control group of 42 patients from the Euro-Image Research core laboratory database. Interestingly OCT showed smaller lumen dimensions area in the stent thrombosis group. In-fact minimal lumen area and minimal stent area measurements were significantly smaller in presence of thrombosis (p < 0.001 and p = 0.03, respectively). Furthermore, in the group with subacute thrombosis, procedurerelated problems such as under expansion, edge dissection, and reference lumen narrowing were significantly more frequent. Globally, features indicative of suboptimal stent deployment occurred in 20 (95.2%) of 21 patients with ST versus 18 (42.9%) of 42 in control group (P = 0.0003).
Such findings with OCT were not unexpected. In-fact consistent with this finding, the large Intra-Vascular-UltraSound (IVUS) substudy of the ADAPT-DES (Assessment of Dual Antiplatelet Therapy with Drug Eluting Stents) showed that the presence of attenuated plaque, tissue protrusion, reference segment plaque burden, and edge dissection are significant predictors of stent thrombosis [9].
OCT, with its high resolution shows features that may be missed by IVUS and is therefore very instrumental to verify optimal stent deployment. Features such as malapposition, intra stent plaque/ thrombus protrusion, or dissections at the stent edges and inside the stents are easily identified by OCT [10-12]. The CLI-OPCI studies [13,14] were specifically designed to further understand the clinical impact of OCT features of suboptimal stenting, pointed out by the CLI-THRO study.
The CLI-OPCI II14 addressed the role of OCT findings after PCI in a large population comprising 832 patients and 1002 lesions with a median follow-up of 319 days. The CLI-OPCI II showed that OCTdefined suboptimal stent deployment was a relatively common finding (31.0% of cases) with a significantly higher prevalence in patients experiencing major acute cardiac events (MACE) in the first year of follow up (59.2% vs. 26.9%, p<0.001) and was an independent predictor of worse outcome (HR=3.53, p<0.001).
Consistently the multicenter CLI-OPCI study [13] aimed at verifying in 670 patients whether the use of OCT can improve the 1-year composite event of cardiac death or nonfatal myocardial infarction after PCI in a real world population, disclosed adverse features OCT requiring further interventions in 34.7%. Patients who underwent OCT-guided intervention were compared with those from a control group by means of propensity score adjustment. Interestingly OCT guided intervention halved the rate of death and myocardial infarction from 13% to 6.6% (p=0.006).
The study showed that OCT can potentially improve the clinical outcomes after stenting in a real-world population. However, its promising conclusions were approached with caution due to its non-randomized design and relatively small population size.
OCT findings after stenting: What we should correct and what we should not?
The CLI-THRO [8] and CLI-OPCI II [14] studies identified quantitative metrics to indicate good stent positioning.
Incomplete lesion coverage and residual reference segment stenosis (Figure 1) is by far the most important metric of nonadequate stent deployment. Of note it has been also associated with stent thrombosis in IVUS studies [9]. In the CLIO-PCI II study, stented segments exhibiting a narrowing at the reference (lumen area <4.5 mm2 in the presence of significant plaque) experienced a worse outcome with a risk of MACE approximately five times higher regardless of the location (proximal or distal reference segment).
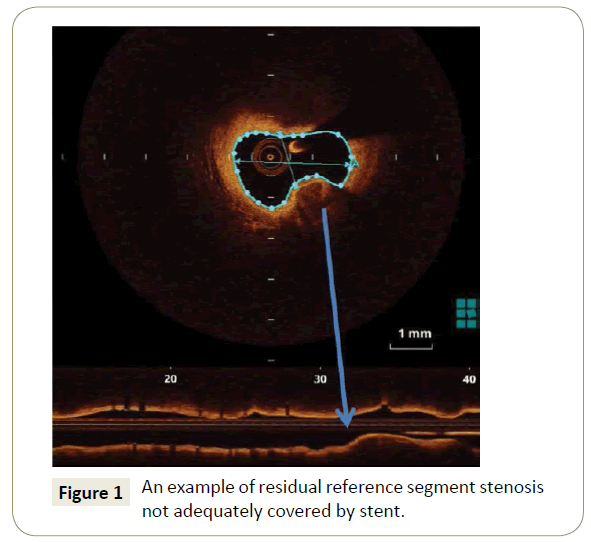
Figure 1: An example of residual reference segment stenosis not adequately covered by stent.
Distal dissections
Dissections >200 μm at the distal stent edge (Figure 2) is an adjunctive feature that conveys an higher risk of MACE (HR 2.54, p=0.004), while proximal dissections had no clinical impact. The same conclusion was reached by Bouki et al. [15]. According to authors, who studied 74 patients with Acute Coronary Syndromes (ACS), presence of a residual dissection flap >0.31 mm, carried an adverse long term clinical impact. The negative clinical impact of stent edge dissection, shown in the CLI-OPCI study, was exerted in the early phase after intervention with the vast majority of MACE occurring during the first 3 months after the procedure. This finding does not contradicts the conclusions reached by Radu et al. [16] who showed in a serial OCT study the late dissection tend to heal at late follow-up: in fact at one year 90% of edge dissections were completely healed on OCT.
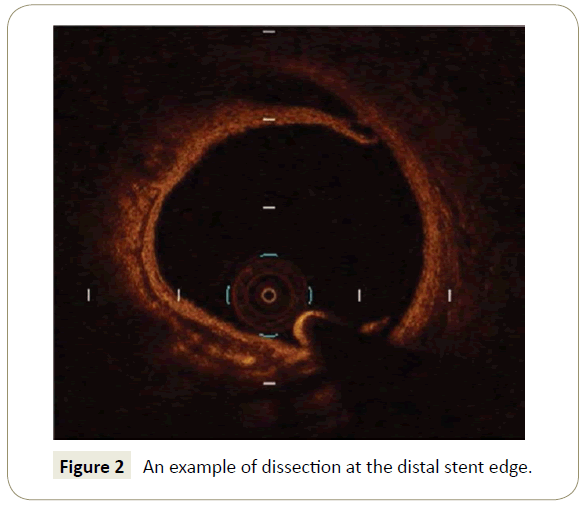
Figure 2: An example of dissection at the distal stent edge.
Small final in-stent MLA (Figure 3), was significantly related to clinical outcome in the CLI-OPCI II14, with a final threshold of 4.5 mm2 that served as the best OCT MACE predictor.
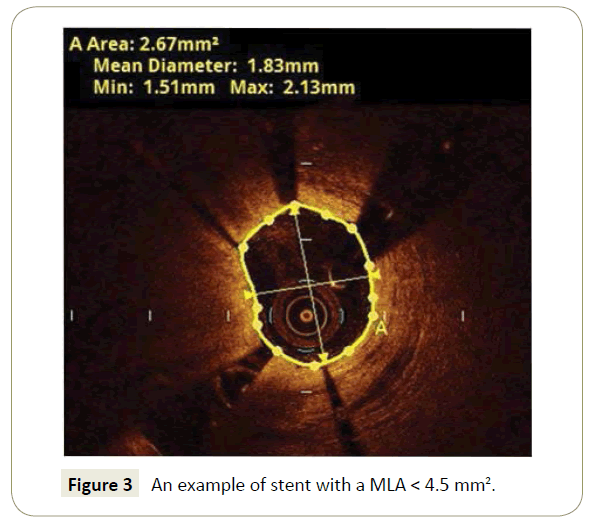
Figure 3: An example of stent with a MLA < 4.5 mm2.
It has been suggested that acute malapposition (Figure 4) may be associated with reduced re-endothelialisation and increased neointima formation. However, our data corroborate IVUS findings [17,18] that failed to relate acute stent-vessel wall malapposition with clinical outcome. Such conclusions were also in line with those that recently emerged from the CLI-THRO study [8] as well as a report from Im et al. [19].
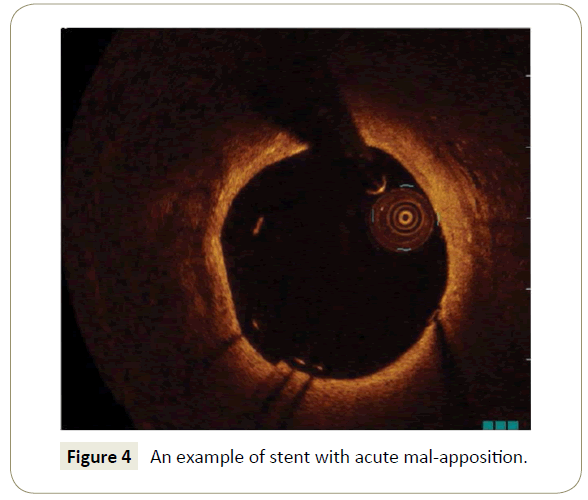
Figure 4: An example of stent with acute mal-apposition.
Intravascular imaging modalities can reveal a wrong stent positioning, missed by angiography. This issue has been stressed by the CLI-OPCI13, showing a 34% of non-optimal stent positioning despite a final look use of OCT, after achievement of a good result at angiography.
The pioneering studies conceived in the CLI-OPCI projects, set up the basis for randomized OCT trails aiming at a comparison between OCT and angio-guided approach to improve stenting. The ILUMIEN III trial [20] showed that OCT guidance of stent deployment significantly increases minimal in-stent area in comparison with the angio-guided control arm. This surrogate end-point was achieved with optimal selection of stent lumen diameter by means of OCT assessment pre-intervention. Post intervention OCT evaluation was of utmost importance to correct stent under expansion and distal dissection consistent with previous studies.
Conclusion
There are strong evidences that intravascular imaging modalities play a key role in improving PCI. OCT is particular helpful as can depict plaque components and stent features at high resolution. The CLI-OPCI studies and the ILLUMIEN trials underlined the role of OCT to guide interventional procedures, showing that the identification and the correction of some findings indicative of suboptimal stent positioning (in stent MLA < 4.5 mm2, edge dissections and residual reference segment stenosis), can improve clinical outcome reducing the incidence of MACE during the follow-up.
References
- Kang SH, Chae IH, Park JJ, Lee HS, Kang DY, et al. (2016) Stent thrombosis with drug-eluting stents and bioresorbable scaffolds: Evidence from a network meta-analysis of 147 trials. JACC CardiovascInterv 9: 1203-1212.
- Palmerini T, Benedetto U, Biondi ZG, Della Riva D, Bacchi RL, et al.(2015) long-term safety of drug-eluting and bare-metal stents: evidence from a Comprehensive Network Meta-Analysis. J Am CollCardiol 65: 2496-507.
- Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, et al. (2012) Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC CardiovascInterv 5: 12-20.
- Fujii K, Carlier SG, Mintz GS, Yang YM, Moussa I, et al. (2005) Stent under-expansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am CollCardiol 45: 995-998.
- Liu J, Maehara A, Mintz GS, Weissman NJ, Yu A, et al. (2009) An integrated TAXUS IV, V, and VI intravascular ultrasound analysis of the predictors of edge restenosis after bare metal or paclitaxel-eluting stents. Am J Cardiol 103: 501-506.
- Kang SJ, Cho YR, Park GM, Ahn JM, Kim WJ, et al. (2013) Intravascular ultrasound predictors for edge restenosis after newer generation drug-eluting stent implantation. Am J Cardiol 111: 1408-1414.
- Song HG, Kang SJ, Ahn JM, Kim WJ, Lee JY, et al. (2014) Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheter CardiovascInterv. 83: 873-878.
- Prati F, Kodama T, Romagnoli E, Gatto L, Di Vito L, et al. (2015) Suboptimal stent deployment is associated with subacute stent thrombosis: optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators: The CLI-THRO study. Am Heart J 169: 249-256.
- Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, et al. (2014) Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation 129: 463-470.
- Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, et al. (2012) International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am CollCardiol 59: 1058-1072.
- Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, et al.(2012) Expert's OCT Review Document. Expert review document part 2: Methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J 33: 2513-2520.
- Di Vito L, Yoon JH, Kato K, Yonetsu T, Vergallo R, et al. (2014) COICO group (Consortium of Investigators for Coronary OCT). Comprehensive overview of definitions for optical coherence tomography-based plaque and stent analyses. Coron Artery Dis 25: 172-185.
- Prati F, Di Vito L, Biondi-ZG, Occhipinti M, La Manna A, et al. (2012) Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: The Centro per la Lottacontrol'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroInterven 8: 823-829.
- Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, et al.(2015) Clinical impact of OCT findings during PCI: The CLI-OPCI II Study. JACC Cardiovasc Imaging 8: 1297-1305
- Bouki KP, Sakkali E, Toutouzas K, Vlad D, Barmperis D, et al. (2015) Impact of coronary artery stent edge dissections on long-term clinical outcome in patients with acute coronary syndrome: An optical coherence tomography study. Catheter CardiovascInterv 86: 237-246
- Radu M, Raber L, Heo J, Gogas B, Jorgensen E, et al. (2014) Natural history of optical coherence tomography-detected non-flow-limiting edge dissections following drug-eluting stent implantation. EuroInterv 9: 1085-1094.
- Hong MK, Mintz GS, Lee CW, Park DW, Park KM, et al. (2006) Late stent malapposition after drug-eluting stent implantation: an intravascular ultrasound analysis with long-term follow-up. Circulation 113: 414-419.
- Tanabe K, Serruys PW, DegertekinM, Grube E, Guagliumi G, et al. (2005) TAXUS II Study Group. Incomplete stent apposition after implantation of paclitaxel-eluting stents or bare metal stents: insights from the randomized TAXUS II trial. Circulation 111: 900-905.
- Im E, Kim BK,Ko YG, Shin DH, Kim JS, et al. (2014) Incidences, predictors, and clinical outcomes of acute and late Stent Malapposition Detected by Optical Coherence Tomography After Drug-Eluting Stent Implantation. CircCardiovascInterv7: 88-96
- Ali ZA, Maehara A, GEnEreux P, Shlofmitz RA, Fabbiocchi F, et al (2016) Ilumien Iii: Optimize PCI Investigators.Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): A randomised controlled trial. Lancet 388: 2618-2628.