- (2006) Volume 7, Issue 2
Mohammed Abu-Hilal, Mark JW McPhail, Lucy Marchand, Colin D Johnson
University Surgical Unit, Southampton General Hospital. Southampton, United Kingdom
Received: 14 October 2005 Accepted: 25 November 2015
Context Release of oxygen free radicals is increased in acute pancreatitis, but whether this can be used to predict clinical severity is not known. Objective This study assesses whether plasma concentrations of malondialdehyde (a marker of lipid peroxidation) and superoxide dismutase (an oxygen free radical scavenger) can be used to predict severity of acute pancreatitis. Patients Fifty-one patients with acute pancreatitis and two control groups were recruited. Main outcome measures Plasma levels of malondialdehyde and erythrocyte content of superoxide dismutase were measured at 0, 12, 24, 48, 72, 96 and 120 hours after admission. Acute physiology and chronic health evaluation (APACHE) II, Glasgow and Ranson scores were calculated. Acute pancreatitis severity was defined by Atlanta criteria. Premorbid antioxidant intake was assessed by dietary questionnaire. Results Levels of malondialdehyde were raised in acute pancreatitis patients and increased in patients with severe compared with mild acute pancreatitis; 12 hours after admission plasma malondialdehyde was 4.42±0.54 mmol/L and 2.95±0.24 mmol/L in severe and mild pancreatitis, respectively (mean±SEM; P=0.007). Plasma malondialdehyde greater than 2.75 mmol/L at 12 hours after admission had high overall accuracy for predicting severe acute pancreatitis. Superoxide dismutase levels were found to decrease in acute pancreatitis but no substantial significant difference was demonstrated between severe and mild acute pancreatitis patients. There was no difference in pre-morbid antioxidant dietary intake between the mild and severe pancreatitis groups. Conclusion Plasma malondialdehyde may be a helpful additional marker of severity in the very early stages of acute pancreatitis.
Antioxidants; Malondialdehyde; Oxidative Stress; Pancreatitis; Superoxide Dismutase
MDA: malondialdehyde; OFR: oxygen free radicals; SOD: superoxide dismutase
Acute pancreatitis can present as a wide clinical spectrum ranging from mild, selflimiting localized disease to fatal widespread multi-organ failure in which mortality ranges from 14 to 30% [1, 2]. Accurate prediction of disease severity may help identify a subgroup of patients who will benefit from intensive monitoring and management, and thus improve outcome.
Prediction of severity in acute pancreatitis usually relies on multiple factor scoring systems, either pancreatitis-specific [3, 4] or the general intensive care system acute physiology and chronic health evaluation (APACHE) II [5]. Markers of the inflammatory response have been investigated in this context: interleukin-8 (IL-8) and IL-6 [6] are predictive, but difficult to measure; Creactive protein (CRP) is well known as a marker of severity [7], but there is often a delay of several days from onset of symptoms before the rise in CRP [8]. Recently, procalcitonin has been proposed as a marker of severity [9, 10, 11, 12, 13], but it may be that this marker is most closely related to the presence of infection [14, 15]. To date, no completely satisfactory mechanism for prediction of severity in acute pancreatitis has been developed and any additional information on severity, especially early in the course of the disease may be helpful.
Oxidative stress has a primary role in the pathogenesis of acute pancreatitis [16, 17, 18, 19, 20]. It occurs when oxygen free radicals (OFR) are produced in quantities too numerous to be scavenged by the antioxidant defences [21], which include the enzymes superoxide dismutase (SOD), catalases and glutathione peroxidase [22]. One possible pathway of oxidative stress imbalance in acute pancreatitis may occur when pancreatic protease is involved in the cleavage of xanthine dehydrogenase to form xanthine oxidase [20, 21, 22, 23]. This enzyme converts hypoxanthine (an ATP breakdown product) into xanthine and superoxide radicals [24]. OFRs lead to damage of phospholipids in cell membranes by the process of lipid peroxidation [20]. This releases toxic stable decomposition products such as malondialdehyde (MDA) which may increase cellular injury or which may act as a chemoattractant to trigger the systemic inflammatory response syndrome (SIRS) involving the complement cascade, various cytokines and other acute phase substances [25]. This raises the possibility that MDA may be a marker of severe pancreatitis, as a result of release following oxidative injury, and as a participant in the events leading to SIRS.
The aims of this study are to evaluate the behaviour of an endogenous OFR scavenger (SOD) and a product of the lipid peroxidation phenomenon (MDA), to consider their potential role for predicting the severity of acute pancreatitis and to assess the difference in premorbid antioxidant dietary intake between mild and severe pancreatitis.
Patients
Patients with acute pancreatitis of any aetiology were recruited into the study with no age limits. Fifty-one patients were studied. The diagnosis was established on the basis of a plasma amylase greater than three times the upper limit of normal and a clinical picture consistent with acute pancreatitis.
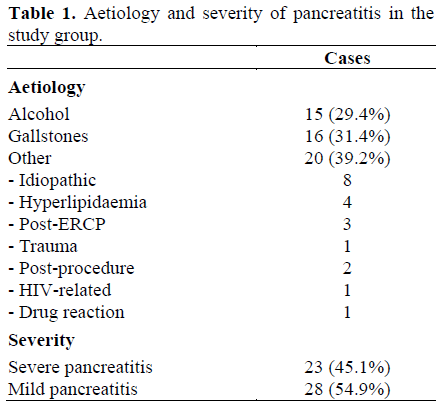
The age of patients with pancreatitis was 55±19 years (mean±SD); there were 30 (58.8%) men and 21 (41.2%) women. Mean preadmission pain duration was 15.8 hours (range: 1-96) with no significant difference between severe (mean 17.8 h, range: 1-48 h) and mild (mean 14.0 h, range: 1-96 h) acute pancreatitis (P=0.453). Clinical features of the patients with acute pancreatitis are shown in Table 1. Twenty-three (45.1%) patients developed complications and were defined as having severe disease. The median number of organ failures scores in patients with severe pancreatitis was 1 (range: 1-3).
Controls
Two control groups were also recruited. First, nineteen patients with acute abdominal pain: disease controls with acute appendicitis (n=3), gastritis (n=8), cholecystitis (n=5) or peptic ulcer disease (n=3). Second, 39 healthy controls with no other condition which might affect the level of free radicals.
Data Collection
Demographic details were recorded on a proforma together with admission plasma amylase level, past medical history, body mass index (BMI), APACHE II (worst score in the first 24 hours of admission [26]). Ranson [4] and Glasgow [3] scores over the first 48 h, duration of hospital stay and computed tomography (CT) findings were recorded. CT was performed only in cases of persisting symptoms one week after admission. All patients underwent abdominal ultrasound. Severity of acute pancreatitis was determined using the Atlanta criteria [27].
Pancreatitis patients and disease controls had blood samples taken on admission (time=0) and at 12 h, 24 h, and daily for five days. Healthy volunteers had only one blood sample measured. Blood was collected in EDTA-containing specimen tubes and centrifuged at 1,440 g for 10 minutes at 4°C. Erythrocytes and plasma were then stored separately at -80°C.
All acute pancreatitis patients were asked to complete a short dietary questionnaire (self and/or interviewer administered) designed to assess their pre-morbid intake of antioxidants in the month prior to presentation. An antioxidant score was produced depending on the frequency of intake of particular foods. These scores were compared between mild and severe groups.
Assays
Erythrocytes and plasma were not available for 11 participants due to laboratory handling error.
Plasma Malondialdehyde
Concentration of MDA in plasma was assayed using a chromogenic assay (Bioxytech LPO-586, Oxis International Inc., Portland, Oregon, USA). This assay measures free and protein-bound MDA without undue interference from other lipid peroxidation products. Standard curves for the range 0-20 μmol/L were prepared for each assay using the chromgen supplied in the kits; the assay has a detection limit of 0.1 μmol/L and an interassay variation of less than 5%.
Erythrocyte Superoxide Dismutase
Erythrocyte lysate from 40 participants was assayed using SOD-525 kits (Bioxytech SOD- 525, Oxis International Inc., Portland, Oregon, USA). This assay is based on an increase in auto-oxidation of a chromogenic reagent by SOD to yield a chromophore which was stable for three minutes and had a maximum absorbance at 525 nm. Standard curves were prepared corresponding to SOD concentrations 0-16 units/mL; samples were diluted at least 1:8 to minimise interference from ascorbic acid and NADPH.
ETHICS
Ethical approval was gained from the university local ethics committee and no patients were recruited until this approval was granted. Patient inclusion in the study was dependent on the ability to give written consent and it was made clear to patients that they may leave the study without compromising their care at any point. The study protocol conforms to the ethical guidelines of the "World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964.
STATISTICS
Data are reported as median, ranges, and mean±SEM (or mean±SD for patient age). Comparison between acute pancreatitis patients and disease controls of mean MDA and SOD levels at each time point (0, 12, 24, 48, 72, 96, and 120 hours) was carried out across all four patient groups (severe and mild pancreatitis, acute abdomen, healthy volunteers) using one-way analysis of variance. A P-value of less than 0.05 was taken as significant. The Fisher’s exact test was applied to discrete data. Associations with severity were examined using a receiver operating characteristic (ROC) curve, and the most discriminating cut-off point was determined by a likelihood ratio (LR) method [28]. Data were analyzed by means of the SPSS/PC 14.0 for Windows and MedCalc v8.1.1. Sensitivities, specificities, and the percentages of cases correctly classified for the various predictors of severity were then calculated.
Plasma Malondialdehyde
The mean plasma levels of MDA in the healthy controls (1.30±0.19 mmol/L) and disease controls (1.13±0.17 mmol/L) were similar (P=0.504) and significantly lower than in the pancreatitis group at time 0 (3.08±0.31 mmol/L; P<0.001 vs. both healthy and disease controls). MDA in the disease controls remained steady from 0 to 120 h (Figure 1). In severe acute pancreatitis, MDA levels rose to a peak of 4.42±0.54 at 12 h after admission, then declined progressively over the following 5 days. In mild acute pancreatitis MDA levels started to decline from 0 h (Figure 1). At 12 hours after admission, plasma MDA significantly (P=0.007) differed between severe (4.42±0.54 mmol/L) and mild (2.95±0.24 mmol/L) pancreatitis.
Figure 1. Comparison of plasma malondialdehyde (MDA) concentration (mean and standard error) in disease controls and in mild and severe acute pancreatitis from 0 to 120 hours after admission. (P values between mild and severe acute pancreatitis; in healthy controls, plasma MDA was 1.30±0.19 μmol/L).
The ROC curve showed a good accuracy of MDA plasma levels at 12 hours in differentiating between mild and severe acute pancreatitis (AUC: 0.765±0.076 SE; Figure 2) and a best cut-off level of 2.75 mmol/L MDA was detected (LR=2.66 at the best cut-off). Sensitivity, specificity, and the percentage of cases correctly classified of MDA, APACHEII and the Glasgow and Ranson scores are shown in Table 2. Negative and positive predictive values could be calculated on the studied sample, but they would be difficult to compare with standard rates due to the high prevalence of severe cases in the studied population.

Erythrocyte Superoxide Dismutase
The mean level of erythrocyte SOD on admission was not significantly different (P=0.499) between severe (551±31 units) and mild (524±25 units) acute pancreatitis, but it was significantly lower than in the healthy control group (678±34 units; P=0.009 vs. severe AP, P=0.001 vs. mild AP). In mild pancreatitis, levels of SOD rose gradually over 5 days (Figure 3). In the severe group the lowest level was observed 24 h after admission and then started to rise possibly more steeply than in mild pancreatitis. However, there was no significant difference between mild and severe acute pancreatitis groups at any, but one, time point during the study: a slightly (P=0.044) higher activity was observed 72 h after admission in severe acute pancreatitis patients; therefore, no predictive values of SOD for severity were calculated.
BMI and Antioxidant Score
No significant difference was noted between the mild and severe pancreatitis groups for duration of preadmission pain, high BMI or pre-morbid antioxidant intake (Table 3).

This study has shown that in clinical acute pancreatitis there is evidence of significantly greater release of MDA in severe cases, compared with mild disease. This is a potential marker of severity for use within 12 hours of admission to hospital. This study suggests that the oxidative stress which leads to MDA formation and release is a reflection of the severity of pancreatic injury, rather than a consequence of anti-oxidant deprivation, because the mild and severe groups had similar antioxidant intakes and similar levels of SOD.
Notable markers of oxidative stress include MDA, which is associated with lipid peroxidation and myleoperoxidase which is associated with primary cytosolic granules within polymorphonucleocytes and is produced during the “oxidative burst” normally used for digestion of bacterial products.
Superoxide ions are potentially harmful; they can be removed by a number of scavenger mechanisms. SOD is one such preferential superoxide scavenger. Decreased serum levels represent consumption of SOD during increased OFR activity. Recent studies in mice have shown that intervening in ceruleininduced pancreatitis with SOD mimetics can reduce amylasemia, pancreatic injury, myleoperoxidase and MDA levels and also reduces mortality [17, 29].
Despite severity scores being available for over thirty years, predicting severity early in the clinical course of acute pancreatitis is still a major challenge. This study utilizes new theoretical and experimental evidence for the important role of oxidative stress markers in predicting severity. Previous experimental [17, 30, 31] studies showed increments of lipid peroxidation products such as MDA in plasma, presumably due to pancreatic tissue damage by OFRs. This has been supported by our data which show statistically significant increases in MDA levels in patients with acute pancreatitis compared to the controls. The data also suggest that MDA may have potential as a marker of severity especially in the first 24 h in hospital.
MDA levels at 12 h after admission were significantly greater in patients with severe acute pancreatitis compared to those with mild disease (P=0.006). Using a cutoff of 3 μmol/L for plasma MDA to detect severe pancreatitis yields sensitivity and specificity comparable to those seen with the various scoring systems but at a much earlier stage in the clinical course. The scoring systems require at least 24 hours (APACHE-II) or 48 hours (Glasgow and Ranson) after admission to acquire all the necessary information. If confirmed, our data suggest that a single measurement of MDA within 12 h of admission could provide similar accuracy. This accuracy does not yet improve on that of marker combination for IL-10 and serum calcium for prediction of organ failure in acute pancreatitis [32].
The clear difference in MDA levels in severe compared to mild acute pancreatitis is consistent with either a process in the severe cases resulting in greater burst of OFR release and hence a greater rise in lipid peroxidation products or, a similar level of OFR but with greater tissue damage in the presence of other unknown factors. The lack of any difference between mild and severe disease of SOD activity at 12 hours supports the latter hypothesis that factors other than OFR release participate in the production of MDA in severe pancreatitis. The factor affecting severity does not appear to be dietary deficiency of antioxidants, which was the same in both groups; although we recognize that our dietary questionnaire provides a relatively crude indication of dietary intake. Although the questionnaire had been used and verified between public health and dietetic departments the use of self-administered questionnaires reduces the impact of this finding.
Schoenberg et al. [17] demonstrated a similar peak in the MDA concentration within pancreatic tissue at 3.5 hours after induction of acute pancreatitis with cerulein infusion. Smaller sampling time intervals in a clinical study are required to decide the optimal time after admission to measure MDA concentration to maximize the statistical power of this important possible predictor of severity.
Mean SOD activity was found to decrease in patients with acute pancreatitis in line with previous findings [23, 24, 33, 34]. No clear early statistical difference could be observed between mild and severe acute pancreatitis groups that could lead to a use for this test in predicting severity. This corroborates a result from a recent smaller study of 13 patients [35] that was unable to demonstrate a difference in SOD levels between mild and severe acute pancreatitis groups in samples taken at recruitment. We use erythrocyte SOD in keeping with available assays but this negative result may reflect the fact that the assay does not test for the pancreatic SOD possible in animal studies.
Analysis of marker differences and predictive power for subgroups of the Atlanta criteria are not possible given the small numbers of patients in this study, but is important to test our hypotheses. A larger study prospectively designed for this purpose would be required. In conclusion, this study found increased levels of MDA within 12 hours of admission to hospital. Erythrocyte SOD activities were similar in the two categories. This suggests that MDA may result from intrapancreatic oxidative stress, and plasma MDA may be an early indicator of severity in pancreatitis, with similar accuracy of severity prediction to currently used scoring systems. A larger, prospective trial is required to further quantify the time-dependence, inter subgroup differences, and appropriate cut-off points for this important marker of oxidative stress in acute pancreatitis.