Original Article - (2018) Volume 19, Issue 4
Department of Surgical Gastroenterology, Madras Medical College, Chennai-600003
Received April 25th, 2018 - Accepted July 25th, 2018
Background Lympho Epithelial Cysts are rare benign lesions, mostly seen involving the tail of pancreas in middle aged men. But precious little is known of these cysts as most are described in case reports only. So a comprehensive analysis of these case reports is needed to glean useful information and form an algorithm for effective treatment. Methodology An analysis done of all available case reports in literature reveals most are symptomatic though asymptomatic cases are increasingly detected on routine imaging. Usually solitary, they are mostly multilocular with a mean size of 4.7 cm. The most common markers include fluid carcinoembryonic antigen and serum CA 19- 9. Both can be markedly elevated in lympho epithelial cysts. Conservative wait and watch management can be followed if the patient is asymptomatic and diagnosis is certain. However, only 11.5% underwent such a management revealing an inability to rule out malignancy beyond reasonable doubt or symptomatology of these lesions in most instances. Pancreas preserving enucleation and pancreatic resection procedures are most commonly performed in lympho epithelial cysts depending on the accuracy of diagnosis with an enucleation of the cyst being preferred for those with a definite diagnosis of lympho epithelial cysts. Conclusion Lympho epithelial cysts which are asymptomatic with an accurate preoperative diagnosis can be followed by surveillance. Pre operatively diagnosed symptomatic lesions can be managed by pancreatic parenchyma preserving over radical pancreatic procedures. For patients with doubtful diagnosis, radical pancreatic resection may be necessary. Tumor markers like serum CA 19-9 and fluid carcinoembryonic antigen are most commonly elevated in lympho epithelial cysts further confounding the diagnosis in equivocal cases. Further diagnostic clarity is needed in this subgroup of patients to clearly characterize these lesions. But till then, a low threshold for surgery is required in these patients for effective management.
Carcinoembryonic Antigen; Cysts; Pancreas
CEA carcinoembryonic antigen; CECT contrastenhanced computed tomography; EUS Endoscopic Ultrasound; LEC lympho epithelial cysts
Lympho Epithelial Cysts of the pancreas are benign, slow growing lesions in the pancreas. Due to their rarity, they have been described most often in individualised case reports in medical literature. The aim of this article is to analyse these rare cysts in detail so as to form meaningful conclusions for further treatment and management.
A thirty-three-year-old female patient presented with history of vague abdominal pain and backache for 6 months duration. The pain was confined to the epigastric region with no radiation of pain. There were no other symptoms. Patient had previous history of emergency splenectomy done 20 years back for trauma. Other medical records pertaining were unavailable. CECT abdomen (Figures 1, 2) done revealed a homogenous cystic lesion at the tail of the pancreas. Ultrasound guided aspiration of the cyst revealed a paucicellular, straw coloured infiltrate, negative for malignant cells. Fluid CEA-2ng/mL (N<2.5 ng/mL), F. CA19-9 was 148 IU/mL (N-<37 IU/mL). Serum CA19-9 and S.CEA were normal. In view of persistent abdominal pain and increased fluid CA19-9, it was decided to proceed with surgery. Intraoperatively, 5 cm smooth walled cystic lesion was seen in the tail of pancreas. As the lesion was adherent to the tail of the pancreas without a clear plane of separation from the pancreas and as malignancy could not be conclusively ruled out, it was decided to proceed with distal pancreatectomy (Figure 3). Cut section of the cyst revealed a thick greasy material with smooth wall. Histopathology of cyst revealed stratified squamous epithelium with overlying layer of lymphoid tissue. This was consistent with a diagnosis of lymphoepithelial cyst of pancreas. Post operative course was uneventful.
All cases of lymphoepithelial cysts described in literature were analysed after a thorough search of medical literature and databases like PUBMED. Though most cases described are case reports, there are a few recent case series of the same also. All reports were divided into three periods based on two landmark publications by Adsay et al. [1] in 2002 and by sekwani et al. [2] in 2010.
Our aim was to analyse the incidence, geographical location, patient variables like age, sex, symptoms, location of cyst in pancreas including type, treatment offered and follow up of Lymphoepithelial cysts (LEC)of pancreas based on the three periods to observe for changes in management over time.
Based on our study, we analysed a total of 235 cases (including our case) which were divided into (Figure 4)
Till 2002 (till Adsay et al.) - 82 cases
2003-2010 (till Sekwani et al.) - 69 cases
2011- till date (after Sekwani et al. -till date) - 84 cases(including our case)
Over time, it is seen that a there is an increasing trend towards increased non operative “wait and watch” which is made possible due to increased accuracy in diagnosis of LEC. Further information on the methodology and cases considered is in annexure 1.
Inclusion Criteria
All cases of lymphoepithelial cysts in the pancreas and peripancreatic area were included in the study. The diagnosis of LEC in operated cases rested on histopathology which showed a cystic lesion lined by stratified squamous epithelium surrounded by lymphoid tissue in the absence of skin appendages like hair. Though some cases were described previously in literature as “epidermoid cyst derived from an accessory spleen in the pancreas” or “accessory splenic epidermoid cyst”, the pathologic description and illustrations for these cases were suggestive of a lymphoepithelial cyst [3, 4, 5, 6, 7, 8, 9, 10, 11].
Exclusion Criteria
Other cystic lesions of pancreas including malignancy, benign lesions like dermoid cyst and some early cases reported with incomplete information were excluded.
The following inference could be made on the data analysed.
Incidence
LEC are probably worldwide in distribution with most cases (70.2%) being reported from the United States (n=113, 48.8%) and Japan (n=52, 22.1%) (Table 1). The term “lymphoepithelial cyst” was coined by Troung in 1987 [12]. However, cases suggestive of lymphoepithelial cysts have been described in literature since 1980. The data has been increasing annually over time with most cases reported so far in 2006 (31 cases) (Figure 5). The vast majority (N=108) (45.9%) of reported cases are single case reports with some large case series being reported recently. The largest case series reported so far is by Dalal et al. (16 cases) [13], followed by Adsay et al. [1] (12 cases) with Nasr et al. [14] and Raval et al. (9 cases each) [15].
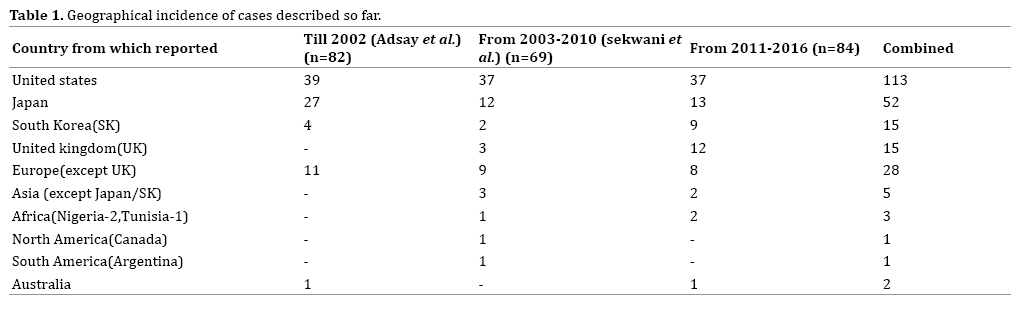
Patient Variables
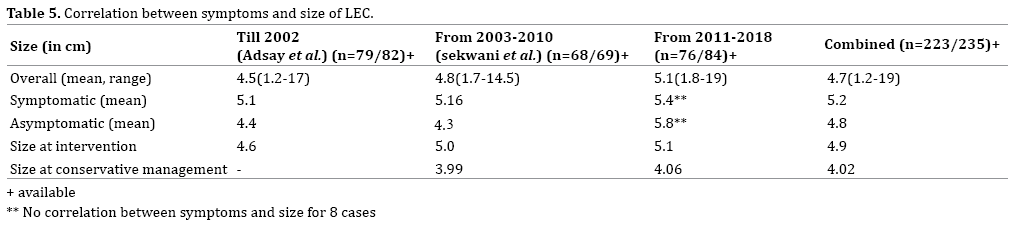
LEC are most commonly seen in the middle aged with a male preponderance of 4:1 (n=189, 80.4%). Overall, LEC are frequently symptomatic (50.2%) (Table 2). The number of asymptomatic cases (40.85%) is rising steadily in world literature due to better accuracy in radiological investigations (Table 3). Among those symptomatic, the most common symptom was abdominal pain (77.9%) in 92 patients followed by weight loss (Figure 6). LEC are usually solitary (n=228) and eccentrically situated within the pancreas (99.1%) (Table 4). The most common pancreatic site is distal to the pancreatic neck where it is most common in the tail (n=98, 41.7%) followed by the body (66, 28.1%) and head (n=64, 27.2%) (Figure 7). Mean size of the cysts is 4.7 cm. Symptomatic cysts had larger diameter compared to asymptomatic cysts (5.2 vs. 4.8 cm) and patients treated by intervention had significantly larger size to those cysts treated by conservative non operative management (4.9 vs. 4 cm) (Table 5). Most common morphological pattern seen is multilocular (48.9%) followed by unilocular cysts (43.8%) (Figure 8).



Interventional Biopsy and Fluid Analysis (EUS/CT Guided)
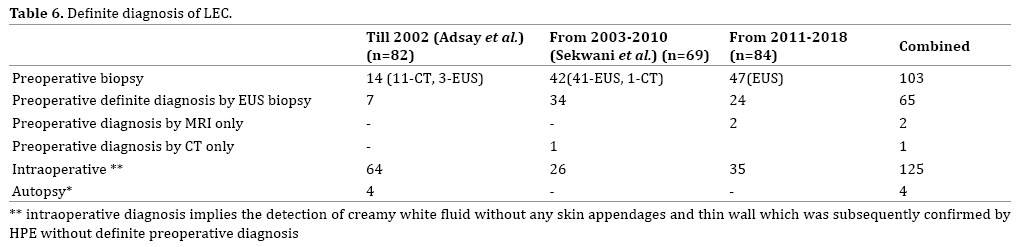
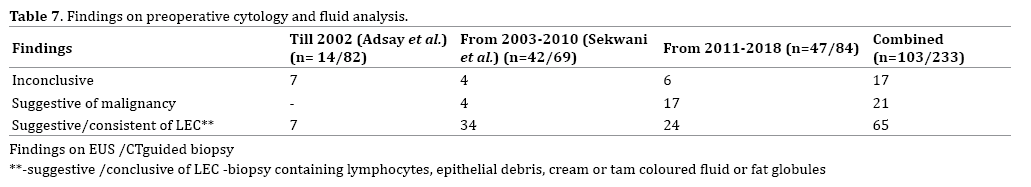
Most cases of LEC are diagnosed preoperatively by EUS guided biopsy and analysis of fluid for tumour markers. Though the incidence of preoperative biopsy is increasing, only 43.8% (N=103) underwent a preoperative biopsy as part of management, with most recent cases undergoing EUS guided biopsy (Table 6). LEC are characteristically acellular, clear, straw –yellow or cheesy white with keratin debris and cholesterol or fat deposition. Jian et al. suggests that FNA helps in accurate diagnosis obviating unnecessary surgery [16]. It is seen that though EUS guided biopsy helps to conclusively diagnose LEC, it may be inconclusive in many. EUS guided trucut biopsy has been suggested for those with equivocal results on EUS FNA to increase the yield [17].
The findings on EUS may be suggestive of LEC, suspicious of malignancy or inconclusive (Table 7).
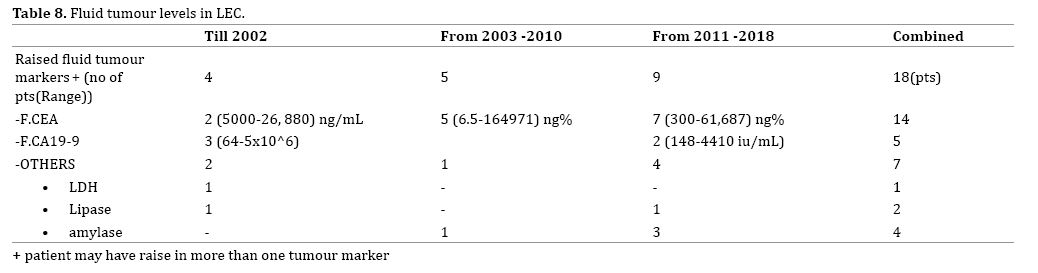
Fluid CEA levels are the most commonly elevated fluid tumour marker where a tumour marker of the fluid was done (Table 8). CEA levels may often be elevated due to goblet cells or the aberrant immunoreactive squamous epithelial lining [15]. Therefore an algorithmic approach for diagnosis of mucinous neoplasm with no solid component on EUS and cyst fluid CEA level of more than 200ng/mL, may not hold good if LEC is also borne in mind [18].
CT Imaging
CT imaging is associated with varied presentations. This may range from characteristic well circumscribed, low attenuating masses with enhancing rims to lobulated, non-enhancing and sharply demarcated lesions with focal calcifications. Most lesions are often associated with the absence of pancreatic ductal dilatation or atrophy [19]. Some lesions may also show variability like unilocularity with clear wall enhancements, regions of fat attenuation, papillary projections, small solid components, wall calcification or thin wall enhancement on conventional CT imaging [19, 20].
This variation has led to the argument that threedimensional computed tomography (3D-CT) scan, rather than the conventional scan, may be better suited to differentiate lymphoepithelial cysts from other lesions of the pancreas [21] primarily because of their predominantly extra-pancreatic 3D location and higher precontrast CT attenuation. Moreover, it is to be noted that LEC are seen to be smaller and more frequently micro lobulated than mucinous cystadenomas [22].
MRI
MRI has been proposed as an accurate investigatory tool in case of equivocal findings on EUS. This is primarily due to its characteristic, high signal intensity on T1 and low signal intensity on T2 weighted imaging. Definite MRI characteristics include “cheerio’s ”appearance of multiple central hypo intensity with peripheral hyper intensity in T2 phase [22], profound water restriction on Diffusion weighted imaging (DWI) [23], or slight signal reduction in out-of-phase when compared to in-phase, because of intraregional variations in fat and water [24].
ERCP
ERCP is not useful to diagnose LEC. It is mostly done when other cystic lesions like IPMN are suspected. Most were described in older case reports where an ERCP failed to show any associated abnormality or communication in the pancreatic duct.
Serum Tumour Markers
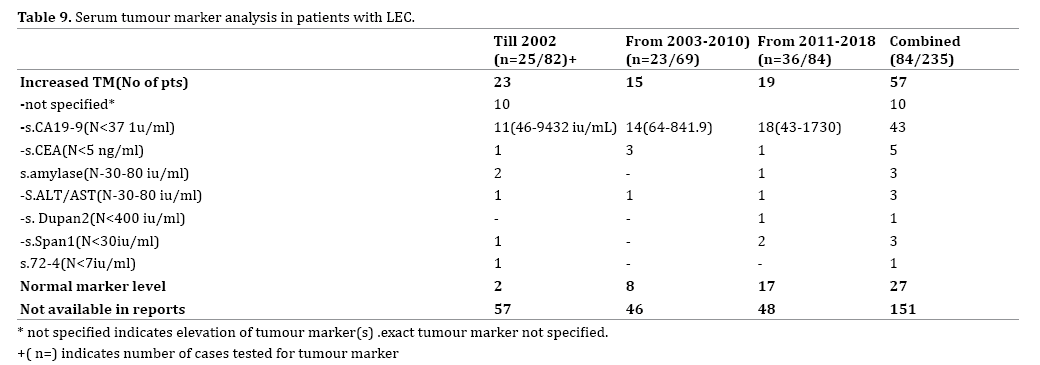
LEC is associated with increased serum and fluid tumour markers which cause diagnostic dilemma especially in equivocal cases. 34.8% (n=82) of cases had serological tumour markers (S.CEA, S.CA19-9 or DUPAN-2) done, of whom 67.07% (n=55) had at least one marker increased beyond normal limits. S. CA19-9 levels were the most common tumour markers elevated with a wide range of values (range-43- 9432 iu/mL). It occurred in 72.7% (n=40) of those with elevated tumour markers followed by S.CEA (range- 5-1582 ng/mL) (Table 9). It is interesting to note that there is a fall in most cases of CA19-9 after surgical excision in patients with elevated CA19-9 levels.
Treatment
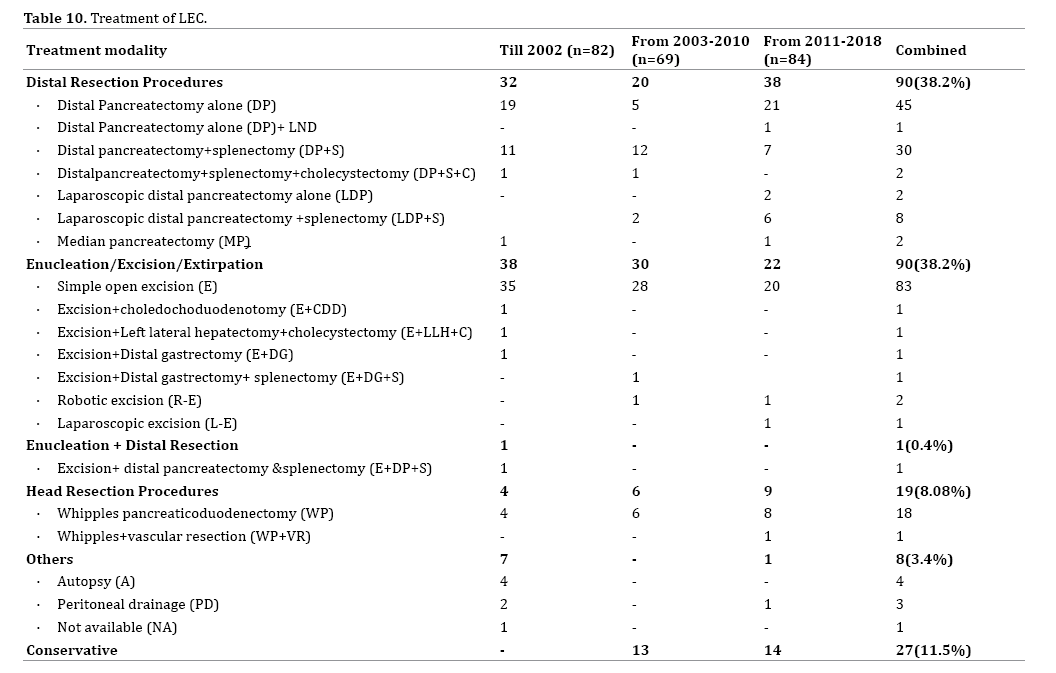
Though a conservative approach is the ideal treatment in asymptomatic preoperatively diagnosed cases of LEC, it was done in only 11.5% of cases (n=27) overall. Most cases of LEC were offered surgery. The indications for surgery include previously diagnosed LEC cysts becoming symptomatic over time, diagnostic uncertainty or suspicion of malignancy. From our analysis, it is seen that local excision of the cyst alone (n=90, 38.2%) and distal pancreatic resection (n=90, 38.2%) were the common procedures performed for symptomatic cysts (Table 10).
Pancreas preserving procedures like local excision, in patients with a definite diagnosis, obviate the need for morbid pancreatic resection. Newer approaches like laparoscopy and robotic surgery have been tried in an effort to further reduce the morbidity of these procedures.
Post Treatment Follow Up
Of all patients on a non-operative “wait and watch” approach, data of the follow up was available for 51.8% (n=14) of these patients with a mean follow up of 26.14 months (range-3-62months). Most of the patients (85.7%) who were on conservative wait and watch reported no increase in size. However, very rarely, increase in size of cyst resulting in symptoms necessitating surgery was seen as was spontaneous resolution [17, 25]. Follow up reports after surgery was available for 39% (n=78) of patients with a median follow up of 7.5 months (range-1 day -125 months). Most common complications after pancreatic resection included pulmonary complications, pancreatic fistula (most common after a distal pancreatectomy) which was usually self-limiting, bleeding, pseudocyst of pancreas, and cyst spillage during surgery.
The gold standard for diagnosis of LEC is histopathology which is quite characteristic with its cheesy, white, greasy porridge like material contained by multiple septations and a thin wall of squamous epithelium with surrounding lymphoid follicles. Microscopy usually reveals absence of skin appendages like hair which is vital to differentiate it from dermoid cyst. Sebaceous differentiation of the epithelium may be present, but is rare [26, 27]. The organism S. Heidelberg et al. has been found in the cyst [28] raising speculation on the presence of infection as a causative factor. However, its exact role is unknown and is not reported in other reports (Figure 9).
Though the exact aetiology of these cysts is unclear, the following hypotheses are considered. Troung’s hypothesis on the histogenesis of LEC include the following [29]:
Proliferation of the ectopic remnant of a brachial cyst in the pancreas. This theory is highly unlikely due to the intraabdominal nature of lesion.
Squamous metaplasia of an obstructed pancreatic duct followed by protrusion into the peripancreatic lymph nodes.
Squamous metaplasia of ectopic pancreatic tissue in a peripancreatic lymph node. This theory is most likely and is supported by evidence [6] including the eccentric location at the pancreas [7] and the occurrence in peripancreatic lymph nodes [10, 29].
Epithelial antigen attraction by lymphocytes induces the differentiation into squamous cells with surrounding lymphocytes [30, 31].
There has been an active interest into whether an association with various viruses like EBV, HIV etc. is present. So far, though there are some case reports of occurrence of LEC in HIV patients, further evidence is lacking [31, 32].
Based on our findings and other reports, a reasonable treatment algorithm for lymphoepithelial cysts of pancreas would be as in figure 9 [15].
Conflict of Interest
Authors declared no conflict of interest and financial disclosure.