Keywords
Overall compliance; Malaria; Lymphatic filariasis; Vector; Morbidities; Endemic diseases
Abbreviation
LGA: Local Government Area; WHO: World Health Organization; WHA66.12: World Health Assembly Provisional Agenda, Item 13.5 (A66/12 11th March, 2013); NTD’s: Neglected Tropical Diseases; LF: Lymphatic Filariasis; DALY’s: Disability Adjusted Life Years; MC: Malaria Consortium; HIV/AIDS: Human Immuno Virus/Acquired Immune Deficiency Syndrome; NMFS: Nigerian Malaria Fact Sheet; ADL: Adenolymphagitis; HH: Health Horizon; CCDC: Centre for Disease Control; TETFUND: Tertiary Education Trust Fund; ASTMH: American Society For Tropical Disease and Hygiene
Introduction
Sustainable for all still a mirage
The World Health Organization (WHO) had been at the fore front of the campaign to realize ‘health for all’ within a stipulated time frame which is also a key aspect of her millennium development goals [1,2]. Unfortunately, this noble objective has become a mirage yet to be captured due to the over reliance on outdated data by various National Health programs of her member states [3-5]. Realizing this gap the WHO in her 66th assembly in May 2013 adopted resolution WHA66.12 which mandated her member states to intensify multifaceted measures and plan investments to improve the health and social well-being of affected populations [6]. Major setbacks to this noble projection include; the incidences of novel infectious diseases and the enigma of Neglected Tropical Diseases (NTD’s) which are viewed as proxies for poverty and the success of interventions aimed at reducing poverty [5]. To realize health for all by the year 2020 which is a projection of previous failed targets calls for sociocultural specific approaches that are feasible to target populations.
It on this premise that the WHO’s focus had shifted from development to sustainable development, from poverty eradication to shared prosperity, and from disease-specific goals to universal health coverage [5]. Recently, the little progress recorded in the battle against NTD’s is being threatened by the menacing effect of climate change which has exacerbated the little grounds gained in the battle against diseases of man and animal. For instance theoretical speculations and real situations show a consistent rise in vector transmitted parasitic diseases restricted to tropical regions in subtropical and high altitude regions of the globe [7-9].
The combined scourge of lymphatic filariasis and malaria in Sub Saharan Africa
Lymphatic Filariasis (LF) and Malaria are regarded as the two most important parasitic diseases in the tropics, collectively infecting more than 500 million people globally and perpetuating poverty and underdevelopment in sub Saharan Africa [3,9-15]. The diseases constitute a great public health scourge worldwide due to the heavy Disability Adjusted Life Years (DALY’s) they elicit on the infected [16,17]. Also, the combined socio-economic burden of LF and malaria is enormous when the direct cost of treatment and losses resulting from physical incapacitation are considered [18-21]. Statistical records indicate that the prevalence of the diseases is nose dividing in sub Saharan Africa but real life cases contradict global and regional statistics due to the numerous undocumented cases in urban and rural areas [22-26]. Studies show that these infections require consistent implementation of practicable measures and practices such as proper body coverage and sanitation, yet their prevalence soars higher than expected.
Malaria affects 3.3 billion people which is about half of the world’s population, in 106 countries and territories. It is estimated that out of the 214 million cases of malaria recorded in 2015, 81% of the cases were in the African region with 438,000 malaria deaths. It is estimated that 91% of all deaths due to malaria were in the African Region, and 86% were children under 5 years of age 27. Globally, malaria is the 3rd leading cause of death for children under five years after pneumonia and diarrhoea [10,12,13,27]. Alarmingly, thirty countries in Sub-Saharan Africa account for 90% of global malaria deaths. Nigeria, Democratic Republic of Congo (DRC), Ethiopia, and Uganda account for nearly 50% of the global malaria deaths. Malaria is the 2nd leading cause of death from infectious diseases in Africa, after HIV/AIDS with about 1 out of 5 deaths of children under the age of 5 Africa associated with malaria [27,28].
An overview of the malaria epidemiology in Nigeria
In Nigeria, malaria is holoendemic with more than 160 million people at risk. The effect of malaria is particularly noticeable in rural areas where malaria frequently strikes during farming seasons [29-33]. In the rural areas of Africa, over one third of primary school children experience clinical symptoms of malaria and are absent from school at least ones weekly in a school term [34-36]. This all year round high incidence of malaria is due to the numerous suitable breeding sites of the malaria vectors particularly during the rainy season [32,37-39]. In Nigeria, high precipitation drives malaria prevalence in addition to factors such as unsanitary environmental conditions, poverty, ignorance, unhealthy habits and inadequate infrastructure [40-42].
An overview of banchroftian filariasis in Nigeria
Lymphatic filariasis (LF) is a disease caused by multiple etiologic agents (nematodes) of which Wuchereria bancrofti is the most economically important due to the chronicity and debilitating nature of the disease it causes. Mosquitoes in the family: Culicidae and genera: Culex Spp, Anopheles Spp and Aedes Spp. are responsible for the transmission of the infection. LF is an accumulative infection occasioned by repeated exposure to microfilariae, affects about 120 million people worldwide [43-47]. Nigeria is the third most endemic country in the world with about 22.1% of the population infected [48]. In approximately 80 tropical and subtropical countries, a billion people are estimated to be at risk of the infection [43,49]. Although, mortality from lymphatic filariasis is low, the disease is the fourth leading cause of permanent disabilities and is second to malaria in terms of Disability Adjusted Life Years (DALYs) [6,43]. The disease results in damages to the lymphatic system leading to lymphoedema, genital pathology and elephantiasis. Clinical manifestation of LF ranges from reoccurring attacks of localized inflammation, tenderness of the lymph nodes and pain often accompanied by fever, nausea and vomiting known as acute adenolymphagitis (ADL), while asymptomatic cases laden with microfilariae (mf) may suffer internal damages to the lymphatic and renal systems [50,51]. Social stigmatization people with LF and the chronicity of the infection aggravate hardship suffered by the infected on a daily basis [32]. Limited knowledge about this disease does not only have a negative impact on the success of control measures but also on health seeking behaviors. Consequently, community health seeking behaviors are influenced by community beliefs and local practices [39,51].
Rationale for the survey
It is pertinent to determine the awareness and attitude of the Nigerian populace towards the two hydra headed giant parasitic infections; malaria and lymphatic filariasis as Nigeria is a huge contributor to the global prevalence of the diseases. The Nigerian ecosystem being conducive for LF spread with due to the efficiency of arthropod vectors and the compatibility status of W. bancrofti well [45,52-55] more people oriented strategies must be adopted to combat the infections. Overcoming the deteriorating sanitary conditions in both urban and rural areas are among the strategies that would drastically reduce the transmission of the disease amongst a myriad of other factors such as health education, distribution of long-lasting insecticidal bed nets, and mass drug administration in Nigeria [23,56]. However, it is envisaged that health education at the grass root and aggressive public enlightenment campaign would be very effective in the reduction of the prevalence of these diseases [57]. The objective of this study is to evaluate the awareness and perception of malaria and lymphatic filariasis in three local Government Areas that constitute the Port Harcourt Metropolis.
Materials and Methods
Study area
The study Area is the metropolitan Port Harcourt city in Rivers State of Nigeria. Port Harcourt city is one of the designated mega cities in Nigeria besides Lagos, Kanu and Abuja with an estimated population of about 6,689,087 by the 2006 National census. It is located geographically within latitude 4, 45’ N, and longitude 6, 50’ E. Rivers State of Nigeria is bounded on the south by the Atlantic Ocean, at the North by Anambra, Imo and Abia States, at the East by Akwa Ibom State and Bayelsa and Delta States at the West 67. The inland part of the State consists of the typical tropical rain forest and mangrove swamps forests at the coastal boundries. Data was collected from the three Local Government Areas (LGA) that make up the Port Harcourt metropolis; Obio- Akpor, Port Harcourt and Eleme LGA’s.
Obio-Akpor LGA is the economic hub of Rivers State because it harbors more than 50% of the private and all the government owned companies. This characteristic makes it a rendezvous for economic and educational activities. Choba community is one of the communities in the Study Area that enjoys the influence of the private and government establishments attracting a huge influx people. Rumuchakara, Owhipa, Okocha, Rumuolaogu, and the University of Port Harcourt community were sampled from Choba sampling Area. The study area lies between latitude 4’ and 5’North and longitude 6’ and 7’East with an estimated population of about 25-30,000 residents. The major occupations of Choba inhabitants are farming, fishing and trading.
Port Harcourt City is the seat of power of the State and harbors all the ministries and headquarters of the major oil companies until recently when Shell moved to Lagos. It is the administrative center of the State. Data was collected from five communities namely; Diobu, Ogbulabali, Nkpoluorworukwo, Rumuibekwe and Rumukalagbo. Port Harcourt LGA covers an area of 360 km square and lies between latitude 4.75’North and longitude 7’East with an estimated population of 1,947,000.
Port Harcourt Eleme which is also one of the study areas is a sub-division of Rivers State Nigeria, located at the east of Port Harcourt. It covers an area of 138 km square meter with a population of 190,884 as at 2006 census. Eleme is geographically located within latitude 4’45’N 7’00’E and longitude 4.75’N 7’E. The main occupations of the people of inhabitants of Eleme include; fishing, farming and trading. Eleme local government area has several communities but the study is restricted to only five communities namely: Alesa, Alode, Ogale, Onne, Okori.
Study population
The sample population consisted of a cross section of people living in three Local Government Areas (Obio-Akpor, Port Harcourt, Eleme). The first group comprised a cross section of Choba residents. The second group consists of people living in five communities in Port Harcourt Local Government Area of Rivers State while comprised of a cross section of people resident in five selected communities in Eleme Local Government Area.
Sampling design/procedure
The Sampling Design used for this research is Cluster sampling. The people were selected randomly in order to avoid bias. The sample size however were 300 people (both male and female) twenty questionnaires were administered in each of the five designated communities, making a total of 300 questionnaires.
Questionnaire administration
The questionnaires administered were used to extract information from the respondents and was done after the study objectives were explained to them. Verbal consent was obtained from respondents following which questionnaires were filled and returned promptly. This included ten major questions to access participants’ knowledge, attitude and perception on the cause, transmission and prevention of malaria and lymphatic filariasis. The respondents that were unable to read the questionnaire were assisted by the researchers to complete the questionnaires.
The questionnaire was pre-tested and modified to address identified needs and additional issues following the format recommended. The additional issues were on the influence of socio-cultural beliefs, psychological implications and consequences of the disease on marriage prospects. Descriptive information was collected through discussions with health officers, community leaders, teachers and students. Specific attention was paid to local terminologies used to describe the manifestation of malaria and lymphatic filariasis in the respective sampling areas.
Data analysis
Quantitative and qualitative data were generated and analysed with SPSS (Statistical Package for Social Sciences) software package.
Results
Characteristics of the study population
The study population comprised 300 respondents from the three Local Government Areas that constitute Port Harcourt metropolis. A total of 100 (33.3%) individuals were targeted for each LGA Data showed that more females responded to questions than males (P>0.05). Age related response showed that the 21-30 years age range responded more to questions 142(29.1%) females and 131(26.8%) males than other age ranges in the study. The 41+ years age range also recorded also recorded low response of 17(3.4%) and 10(2.05%) females and males respectively (Table 1). Amongst the 300 respondents in the 176(58.6%) were single, 109(36.3%) were married and 5(1.5%) were separated. However, the marital status of the respondents was not considered as an associated factor influencing the opinion of the respondents in this the study (Figure 1). There was concentration of singles in Eleme and Obio-Akpor communities which was associated with affordable housing, commerce and security. Port Harcourt LGA had the highest population of married individuals indicative of high and middle class families comprising of mostly civil servants. This study opines that the demographic structure displayed in this study is in tandem with expectations in a city that houses the seat of Government also witnesses a population of civil service personnel families. Again, the heavy industrialization in Eleme and Obio-Akpor LGA’s serve as magnets for young school leavers and entrepreneurs hence the surge in youth population in these areas.
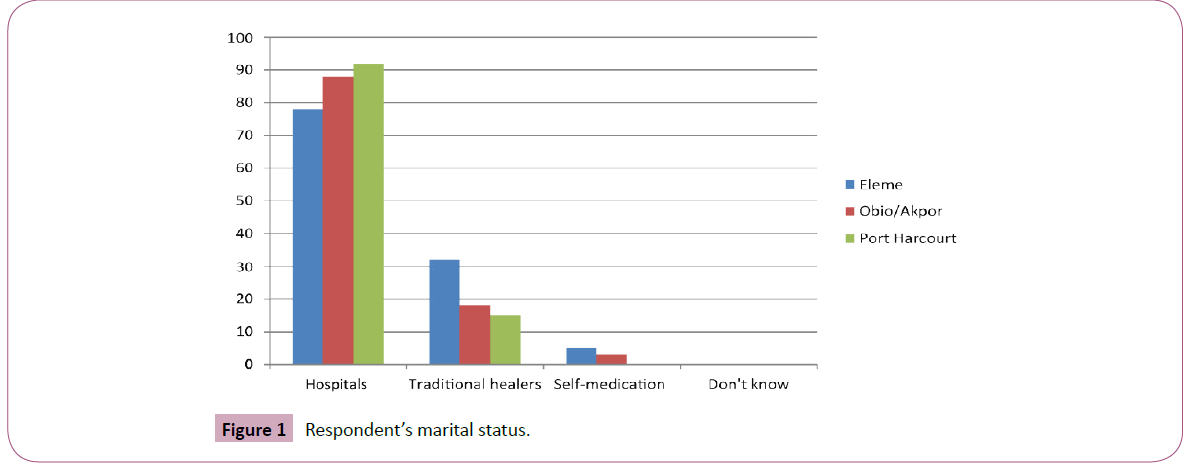
Figure 1: Respondent’s marital status.
| Age Range |
Sex |
Characteristics of the study Population in L.GA’s (%) |
| Fleme |
Obio/Akpor |
Port-Harcourt |
| M |
L.F |
Total |
M |
L.F |
Total |
M |
L.F |
| 14–20 |
Male |
12(52.1) |
11(47.8) |
23(35.9) |
14(66.6) |
7(33.3) |
21(32.8) |
14(70.0) |
6(30.0) |
| |
Female |
11(78.5) |
3(21.4) |
14(36.8) |
6(66.6) |
3(33.3) |
9(23.6) |
9(60.0) |
6(40.0) |
| 21–30 |
Male |
25(56.8) |
18(40.9) |
44(33.5) |
25(73.5) |
9(26.4) |
34(25.9) |
31(58.4) |
22(41.5) |
| |
Female |
28(59.5) |
19(40.4) |
47(33.0) |
33(62.2) |
20(37.7) |
53(37.3) |
23(54.7) |
19(45.2) |
M: Malaria; L.F: Lymphatic Filariasis
Table 1: Characteristics of the study population.
Respondent’s awareness about the diseases
The study observed that awareness of the diseases generally was low considering the endemicity of the two diseases in the study areas (P<0.05). There was variability in opinion amongst the respondents about the two diseases however the awareness of malaria 35.3% amongst the respondents was more than the awareness for Lymphatic filariasis 36.6% Obio/Akpor LGA recorded more awareness for malaria while Eleme LGA recorded more awareness for Lymphatic filariasis (Table 2). The study believes that the increase in awareness level 42.0% of Obio Akpor is associated with the population growth of the area due to its infrastructural evolution and overlap into the Port Harcourt City center which has augmented the flow of information and magnified the level of cultural integration.
| Respondents’ awareness of the disease (%) |
| Awareness |
Eleme |
Obio/Akpor |
Port Harcourt |
Overall Response (%) |
| M |
LF |
M |
LF |
M |
L.F |
| Yes* |
32(86.4) |
5(12.5) |
42(42.0) |
0 |
27(96.4) |
0 |
106 (35.3) |
| No** |
5(6.0) |
33(82.5) |
0 |
42(42.0) |
1(3.5) |
28(28.0) |
110 (36.6) |
| Ignorant |
0 |
2(5.0) |
0 |
0 |
0 |
0 |
5(0.66) |
M: Malaria; LF: Lymphatic filariasis; *0.023553 (p<0.05) and **0.021698 (p<0.05).
Table 2: Respondents awareness of the disease in study areas.
Knowledge about the cause of the diseases
Knowledge about the etiology of the diseases was relatively high in the study especially in Obio Akpor and Port Harcourt study areas. The study reveals that the educational status of the respondents was a contributing factor to this level of awareness. Port Harcourt and Obio Akpor houses a huge chunk of the educational establishment and government ministries which directly influenced the information dissemination. On the other hand, Eleme respondents’ relatively low knowledge about the diseases to relatively poor infrastructural state and the socioeconomic status of the respondents. There was relatively high occurrence of the lack of knowledge about the etiology of filariasis. It was suggests that the ignorance about the filarial infection was due to the infrequent manifestation of clinical symptoms in infected hosts in the study areas. However, the knowledge about lymphatic filariasis was relatively high in Eleme which deviates from what it had been in malaria and the social economic status of the indigenes that has been stressed upon earlier (Table 3).
| Knowledge about the cause of the diseases (%) |
| Knowledge of Cause of Diseases |
|
Eleme |
Obio/Akpor |
Port Harcourt |
| Yes |
94(94.0) |
67(67.0) |
100 (100) |
52(52.0) |
100 (100) |
64(64.0) |
| No |
6 (6.0) |
33(33.0) |
0 |
48(48.0) |
0 |
36(36.0) |
Table 3: Knowledge about the cause of the disease.
Mosquito bite as source of infections
Data shows that most of the respondents in the study areas believed that mosquito bite was the mode of transmission of the diseases. In Eleme LGA, 83.0% of the respondents indicated that mosquito bite was the mode of transmission of malaria while 53.0% indicated the same for lymphatic filariasis. However, in Obio/Akpor LGA, 95.0% of the respondents indicated that mosquito bite was the mode of transmission of malaria, while 25.0% indicated the same for lymphatic filariasis. In Port Harcourt, 90.0% respondents believed that mosquito bite was the means of transmitting malaria while 65.0% indicated the same for lymphatic filariasis.
Sexual intercourse or body contact as source of infections
Data reveals that 12.0% of Eleme respondents indicated that sexual intercourse or body contacts are the mode of transmission of malaria and 19.0% respondents indicated the same for lymphatic filariasis. However, in Obio/Akpor study area some of the respondents believed that food poisoning or stepping on charms is are the means of transmitting the diseases with 6.0% of the respondents for malaria and 34.0% for lymphatic filariasis. In Port Harcourt, 9.0% of the respondents indicated that sexual intercourse or body contact was the means of transmitting malaria while 20.0% respondents indicated the same for lymphatic filariasis.
Food poisoning/stepping on charms as a source of infections
The study revealed a strong influence of superstitious believes on the knowledge and practice of the respondents in the study. It was observed that Eleme LGA which is the study area with the most village or primitive status had the highest misconceptions of about the diseases. However, there was a gradual decline in the misconceptions as the municipality status of the sampling area increased (Table 4). Data shows that 6.0% of respondents from Eleme believed that food poisoning or stepping on charms were the means of transmitting malaria while 34.0% respondents indicated that the same were responsible for the transition of lymphatic filariasis. In Obio/Akpor, 11.0% and 28.0% respondents indicated that food poisoning or stepping on charms were the means of transmitting malaria and lymphatic filariasis respectively. However, 14.0% and 27.0% of the respondents in Port Harcourt indicated that the lymphatic filariarsis and malaria respectively were transmitted through food poisoning or stepping on charms.
| Respondents’ Knowledge on Mode of Transmission of the Diseases (%) |
| Mode of transmission |
Eleme |
Obio/Akpor |
Port Harcourt |
| M |
L.F |
Total |
M |
L.F |
Total |
M |
L.F |
Total (%) |
Sexual intercourse or
Body Contact |
12(12.0) |
19(19.0) |
31(31.0) |
17(17.0) |
16(16.0) |
33(33.0) |
9(9.0) |
20(20.0) |
29(29.0) |
| Mosquito bite |
83(83.0) |
53(53.0) |
136(33.0) |
95(95.0) |
25(25.0) |
120(29.1) |
90(90.0) |
65(65.0) |
155(37.7) |
| Food poisoning or Stepping on charms |
6(6.0) |
34(34.0) |
40(40.0) |
11(11.0) |
28(28.0) |
39(39.0) |
14(14.0) |
18(18.0) |
32(32.0) |
| Witch craft |
0 |
13(13.0) |
13(48.1) |
1(1.0) |
5(5.0) |
6(22.2) |
2(2.0) |
6(6.0) |
8(29.6) |
Table 4: Respondents’ knowledge on mode of transmission of the diseases.
Witchcraft as a source of infections
In Eleme LGA, none of the respondents responded on witchcraft as a mode of transmission of malaria but 13.0% respondents responded to it as a mode of transmission of lymphatic filariasis. However, in Obio/Akpor LGA, 1.0% and 5.0% respondents indicated that malaria and lymphatic filariasis respectively are transmitted through witchcraft, while 2.0% of the respondents from Port Harcourt study area indicated witchcraft as the mode of transmitting malaria while 6.0% respondents indicated the same for lympatic filariasis.
Respondent’s knowledge and experience on the diseases
The study noted that some of the respondents had some experiences about the diseases. Data showed that in Eleme, all the respondents 100% had experienced malaria and 33% individual’s lymphatic filariasis. However, 98.0% of the respondents in Obio/ Akpor indicated suffering from malaria while 20.0% indicated experiencing lymphatic filariasis. Similarly, 99.0% and 25.0% of the respondents in Port Harcourt study area stated experiencing malaria and lymphatic filariasis respectively (Table 5).
| Experiences about infections (%) |
| Experiences or infections |
Eleme |
ObioAkpor |
Port Harcourt |
| M |
L.F |
Total |
M |
L.F |
Total |
M |
L.F |
Total |
| Yes |
100(100) |
33(33.0) |
133(54.4) |
98(98.0) |
20(20.0) |
118(31.4) |
99(99.0) |
25(25.0) |
124(33.0) |
| No |
- |
67(67.0) |
67(29.7) |
2(2.0) |
80(80.0) |
82(36.4) |
1(1.0) |
75(75.0) |
76(33.7) |
Table 5: Respondents’ experiences about the diseases.
Respondent’s knowledge on signs and symptoms of the diseases
Body malaise as a symptom of the infections: Data showed that in Eleme study area 43.0% of the respondents indicated body malaise as a common a symptom of malaria while 4.0% of the respondents stated that body malaise was a symptom of lymphatic filariasis. In Obio/Akpor study area 38.0% of the respondents responded on body malaise as a symptom of malaria while 8.0% respondents indicated the same for lymphatic filariasis. However, in Port Harcourt study area 63.0% of the respondents indicated body malaise a symptom of malaria while 3.0% responded on it as a symptom of lymphatic filariasis (Table 6).
| Respondents’ knowledge on signs and symptoms of the diseases (%) |
| Symptoms |
Eleme |
Obio/Akpor |
Port Harcourt |
| M |
L.F |
Total |
M |
L.F |
Total |
M |
L.F (%) |
Total |
| Abnormal sleep |
23(23.0) |
3(3.0) |
26(32.0) |
40(40.0) |
2(2.0) |
42(51.8) |
12(12.0) |
1(1.0) |
13(16.0) |
| Fever |
35(35.0) |
6(6.0) |
41(43.1) |
32(32.0) |
3(3.0) |
35(36.8) |
18(18.0) |
1(1.0) |
19(20.0) |
| Body malaise |
43(43.0) |
4(4.0) |
47(29.5) |
38(38.0) |
8(8.0) |
46(28.9) |
63(63.0) |
3(3.0) |
66(41.5) |
| Pain |
13(13.0) |
18(18.0) |
31(28.0) |
42(42.0) |
12(12.0) |
54(50.0) |
20(20.0) |
3(3.0) |
23(21.2) |
| Lymph enlargement |
3(3.09) |
28(28.0) |
31(38.0) |
2(2.0) |
38(38.0) |
40(50.0) |
4(4.0) |
5(5.0) |
9(11.2) |
| Itching/scratching |
15(15.0) |
32(32.0) |
47(46.0) |
12(12.0) |
23(23.0) |
14(13.7) |
6(6.0) |
14(14.0) |
20(19.6) |
| Vomiting |
28(28.0) |
9(9.0) |
37(43.5) |
17(17.0) |
6(6.0) |
23(27.0) |
16(16.0) |
9(9.0) |
25(29.4) |
| Others |
5(5.0) |
0 |
5(41.6) |
3(3.0) |
3(3.0) |
6(50.0) |
1(1.0) |
0 |
1(8.3) |
Table 6: Respondents’ knowledge on signs and symptoms of the diseases.
Lymph enlargement as a symptom of the infections: Data revealed that 3.0% respondents in Eleme study area indicated lymph enlargement as a common symptom of malaria while 28.0% respondents indicated lymph enlargement as a symptom of lymphatic filariasis. However, in Obio/Akpor study area 2.0% of the respondent stated that lymph enlargement was associated with malaria while 38.0% responded to it as a symptom of lymphatic filariasis. In the Port Harcourt study area, 4.0% and 5.0% of the respondents indicated that lymph enlargement was a symptom of malaria and lymphatic filariasis respectively.
Abnormal sleep as a symptom of the infection: Data revealed that 23.0% of the respondents from Eleme study area associated abnormal sleep patterns as symptom of malaria while 3.0% respondents associated behavior with lymphatic filariasis. Also, in Obio/Akpor, 40.0% of the respondents associated abnormal sleep patterns as a symptom of malaria while 2.0% respondents believed it was a symptom of lymphatic filariasis. However, 12.0% and 1.0% respondents indicated that abnormal sleep was a symptom of malaria and lymphatic filariasis respectively in Port Harcourt.
Pain as a symptom of the infections: Data showed that in Eleme study area 13.0% and 18.0% of the respondents indicated that body pain as being common with malaria and lymphatic filariasis infections respectively. Similarly, body pain was associated with both diseases in Obio/Akpor study but with variations in the populations that indicated malaria 20.0% and lymphatic filariasis 12.0%. However, in the Port Harcourt study area 20.0% of the respondent also associated pain with malaria while only 3.0% of the respondents related it with lymphatic filariasis.
Fever as a symptom of the infections: Data revealed that 35.0% and 6.0% of the respondents from the Eleme study area indicated fever as a common symptom of malaria and lymphatic filariasis respectively. 32.0% and 3.0% of the respondent from Obio/Akpor study area stated that fever was common with malaria and lymphatic filariasis respectively. However, 18.0% of the respondents from Port Harcourt study area indicated that fever was a common symptom of malaria while 1.0% stated the same for lymphatic filariasis.
Itching and scratching as a source of infections: Data shows that in Eleme study area 15.0% of the respondents indicated that itching and scratching of the body was a symptom of malaria while 32.0% respondents indicated the same for lymphatic filariasis. However, in Obio/Akpor study area 12.0% and 23.0% of the respondents indicated that itching and scratching were symptoms of malaria and lymphatic filariasis respectively. In Port Harcourt study area 6.0% of the respondents indicated that itching and scratching were symptoms of malaria while 14.0% of the respondents indicated the same for lymphatic filariasis.
Vomiting as a source of infections: Data shows that in Eleme study area 28.0% of the respondents indicated that vomiting was a symptom of malaria while 9.0% respondents indicated the same for lymphatic filariasis. However, in Obio/Akpor study area 17.0% and 6.0% respondents indicated that vomiting was a symptom of malaria and lymphatic filariasis respectively. Also in Port Harcourt study area 16.0% and 9.0% of the respondents indicated that vomiting was a symptom of malaria and lymphatic filariasis respectively.
Respondent’s attitude to control measures
Avoidance or abstinence from sexual intercourse: Data showed that in Eleme study area, 8.0% of the respondents indicated that avoidance of sexual contact was a control measure for malaria while 23.0% of the respondents believed the similar disposition was effective for lymphatic filariasis control. Also, 5.0% of the respondents in Obio/Akpor, study area indicated abstinence from sexual activities as effective control measure for malaria while 15.0% indicated the same attitude as effective control strategy for lymphatic filariasis. However, 12.0% and 18.0% of the respondents in the Port Harcourt study area indicated abstinence from sexual intercourse as a control measure for malaria and lymphatic filariasis respectively (Table 7).
| Respondents’ Attitude to control (%) |
| |
ELEME |
OBIO/AKPOR |
PORT HARCOURT |
| Control measure |
M (%) |
L.F (%) |
Total (%) |
M (%) |
L.F (%) |
Total (%) |
M (%) |
L.F (%) |
Total (%) |
| Avoid sexual contact |
8(8.0) |
23(23.0) |
31(38.2) |
5(5.0) |
15(15.0) |
20(24.6) |
12(12.0) |
18(18.0) |
30(37.0) |
| Good personal hygiene |
63(63.0) |
58(58.0) |
121(31.3) |
74(74.0) |
72(72.0) |
146(37.8) |
77(77.0) |
42(42.0) |
119(30.8) |
| Avoid body contact |
- |
22(22.0) |
22(78.5) |
- |
- |
- |
- |
6(6.0) |
6(21.4) |
| Sacrifice to appease God |
2(2.0) |
11(11.0) |
13(20.6) |
7(7.0) |
18(18.0) |
25(39.6) |
4(4.0) |
21(21.0) |
25(39.6) |
| Avoid mosquito bite |
85(85.0) |
37(37.0) |
122(32.8) |
72(72.0) |
43(43.0) |
115(30.9) |
93(93.0) |
41(41.0) |
134(36.1) |
| Avoid eating with an infected person |
- |
2(2.0) |
2(66.6) |
1(1.0) |
- |
1(33.3) |
- |
- |
- |
Table 7: Respondent’s attitude to various control strategy.
Personal hygiene as a control measure of the infections: Data reveals that 63.0% of the respondents in Eleme study area indicated that personal hygiene was a control measure for malaria while 58.8% mentioned the practices as effective measures against lymphatic filariasis. In Obio/Akpor study area, 74.0% and 72.0% of the respondents indicated that personal hygiene was an effective control measure against malaria and lymphatic filariasis respectively. However, in Port Harcourt study area, 77.0% of the respondents indicated that the observance of personal hygiene was a control measure for malaria while 42.0% indicated the same practice an effective strategy for the control of lymphatic filariasis.
Avoidance of mosquito bite as a control measure against the infections: In Eleme study area, 85.0% of the respondents stated that avoidance of mosquito bites was effective against malaria infection spread while 37.0% of the respondents indicated mosquito control as an effective measure against lymphatic filariasis spread. However, 72.0% of the respondents in Obio/ Akpor study area stated that avoidance mosquito bites was a control measure for malaria out of 43.0% of the respondents that stated practice as being effective for lymphatic filariasis. 93.0% and 41.0% of the respondents in Port Harcourt study area stated that avoiding mosquito bite was an effective control measure for malaria and lymphatic filariasis respectively.
Avoid body contact as a control measure of the infections: Data show that none of the respondents in Eleme study area indicated that avoidance of body contact was effective against malaria but 22.0% of the respondents indicated avoidance of body contact as being effective against lymphatic filariasis. In Obio/Akpor study area data reveals that none of the respondents believed that avoidance of body contact was a control measure for both malaria and lymphatic filariasis. However, in Port Harcourt study area none of the respondents believed that avoidance of body contact was a control measure for malaria but 6.0% indicated that it was a control measure for lymphatic filariasis.
Avoidance of eating with an infected person: Data revealed that none of the respondents in Eleme study area responded on avoidance of eating with an infected person as a control measure for malaria while 2.0% respondents believed that avoidance of eating with an infected person was a control measure for lymphatic filariasis. However, in Obio/Akpor study area, 1.0% respondents believed that avoidance of eating with an infected person was a control measure for malaria while none of the respondents believed that for lymphatic filariasis. Also in Port Harcourt study area, none of the respondents believed that avoid eating with an infected person was a control measure of both malaria and lymphatic filariasis.
Sacrifices to gods as a control measure of the infections: Data show that Eleme study area had 2.0% respondents indicating that sacrifice to appease gods was a control measure while 11.0% of the respondents indicated the same for lymphatic filariasis. In Obio/ Akpor study area data revealed that 7.0% and 18.0% respondents indicated that sacrifice to appease gods was a control measure of malaria and lymphatic filariasis respectively. However, in Port Harcourt study area 4.0% respondents indicated that sacrifice to appease gods was a control measure for malaria while 21.0% respondents indicated the same for lymphatic filariasis.
Respondent’s knowledge on socio-economic and physiological consequences
Data showed that the overall total of 161(31.2%) believed that the socio-economic and physiological consequences will increase spending on health on the affected individuals while 128(24.8%) believed that the consequences will lead to loss of self-esteem. 82(15.9%) believed that it hinders daily income while 64(12.4%) believed that it is a way of losing their source of income. 47(9.1%) and 33 (6.4%) respondents respectively believed that avoiding social gathering and absenteeism from school or work are the consequences (Figure 2).
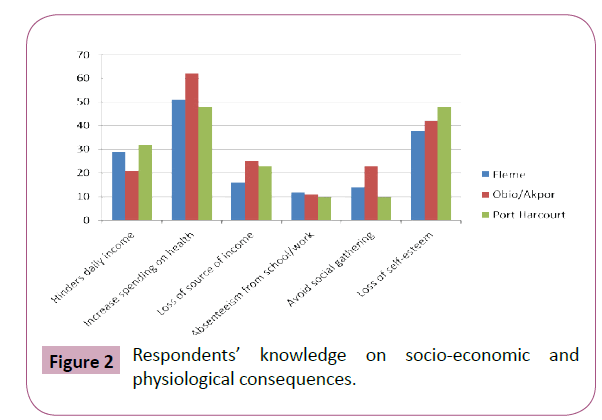
Figure 2: Respondents’ knowledge on socio-economic and physiological consequences.
Respondent’s knowledge on best health care services: Data showed that a good number of respondents from the study areas believed that hospitals is the best health care services for these infections with the overall total of 258(77.9%) followed by traditional healers with 65(19.6%), while 8(2.4%) believed that self-medication is the best health care for these diseases (Figure 3).
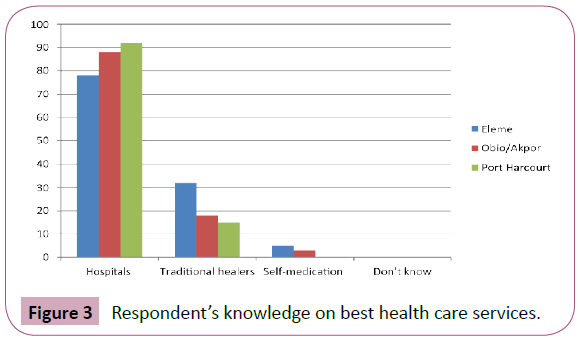
Figure 3: Respondent’s knowledge on best health care services.
Discussion
It was found in this study that there was a high level of awareness (80.3%) among respondents in the Study Areas. The ability of community members to protect themselves from malaria and lymphatic filariasis was based on their access to proper information about the diseases through health education [58-61]. The study reveals that the high awareness level in the study areas may be attributed to the closeness to the Port-Harcourt metropolis which has numerous agents of enlightenment (Figure 4).
Figure 4: Map of study area.
The knowledge of vector species is important in understanding the epidemiology of malaria and Bancroftian filariasis. Majority of the respondents were familiar with malaria and lymphatic filariasis manifestations, however, their awareness on the cause and transmission was poor. This study revealed that preventions were based on superstitious beliefs. These diseases distorted awareness on the true causes of the infections and the control measure is believed to be a primary risk factor in new infections as much of the information about the diseases were based on speculations or mythical beliefs, attributing transmission to witchcraft, sorcery and some evil spell [58-64]. Despite the endemic statuses of the Study Areas (64.5%), of the respondents were aware that the diseases are spread by mosquito bites. The role of mosquito in the transmission of parasitic agents of filariasis was poorly appreciated in many of the communities investigated and thus it was not surprising that there was little awareness of the importance of minimizing mosquito contacts for preventing infections. This result is in agreement with [58,59,62,64].
Respondents in the three local government areas were knowledgeable on the consequences of the diseases in households and the stigmatization that infected people are faced with which hampers economic and marriage prospects and matrimonial stability. Findings from descriptive data suggested that patients with chronic malaria avoided social gatherings which also declined their daily incomes while patients with advanced stages of lymphedema and hydrocele were noted to be isolated. This behavior is highly influenced by community perception on the causes, transmission and prevention of these diseases. Some of these cultural beliefs were due to ignorance and illiteracy [47,58,63,64].
Health education can therefore be designed to persuade the communities and individuals to correct the misconceptions associated with malaria and lymphatic filariasis. This study suggests that the responses from the three Local Government Areas appeared to be more influenced by superstition. It was observed that misconceptions about the infections can adversely affect any meaningful control measures against mosquito. In addition the use of appropriate health facilities and treatments are recommended [65-67].
Conclusion
In view of the active transmission of malaria and lymphatic filariasis in these Local Government Areas, with chances of prevalence, intensity and clinical symptoms increasing over time, there is urgent need for control. It is therefore recommended that more comprehensive epidemiological and entomological studies should be carried out in other communities of the Local Government Areas and the entire state to really establish the disease profile before control.
Acknowledgement
The Department of Animal and Environmental Biology, Faculty of Science, University of Port Harcourt is greatly appreciated for approving the project proposal and the logistics for data collection. We also appreciate the Tertiary Education Trust Fund (TETFUND) for releasing the funds for conference presentation at the annual conference of American Society for Tropical Medicine and Hygiene (ASTMH), Pheladephia 2015.
Author’s Contribution
Sidney O Nzeako designed the study, supervised the collection of data and produced the final draft. Florence O Nduka assisted in the design and proof reading of the final draft. Oyenike H Okunnuga and Chinonye N Ezenwaka collected data and produced the initial draft.
Conflict of Interest
Authors declare no conflict of interest.
References
- van Herten LM, van de Water HP (1999) New global health for all targets. BMJ 319: 700-703.
- Ahorlu CK, Dunyo SK, Koram KA, Nkrumah FK, Aagaard-Hansen J, et al. (1999) Lymphatic filariasis related perceptions and practices on the coast of Ghana: implications for prevention and control. Acta Trop 73: 251-261.
- Onyenekennwa CE (2011) Nigeria’s vision 20; 2020-issues, challenges and implications for development management. J Rural Dev1: 21-40.
- https://www.euro.who.int/__data/assets/pdf_file/0007/147724/wd09E_Health2020_111332.pdf
- WHO (2015) Investing to overcome the global impact of neglected diseases. Third WHO report on neglected tropical diseases: WHO department of control of neglected tropical diseases.
- Wynd S, Melrose WD, Durrheim DN, Carron J, Gyapong M (2007) Understanding the community impact of lymphatic filariasis: a review of the sociocultural literature. Bull World Health Organ 85: 493-498.
- Thomson MC, Connor SJ, Milligan PJ, Flasse SP (1996) The ecology of malaria as seen from earth-observation satellites. Ann Trop Med Parasitol 90: 243-264.
- Gallup JL, Sachs JD (2001) The economic burden of malaria. Am J Trop Med Hyg 64: 85-96.
- The carter centre fighting diseases in Nigeria (2016) Eliminating lymphatic filariasis453 Freedom Parkway, Atlanta, GA 30307, USA.
- Newman RD, Hailemariam A, Jimma D, Degifie A, Kebede D, et al. (2003) Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a non-epidemic year. J Infect Dis 187: 1765-1772.
- Elchner M (2009) Malaria life cycle.
- World Malaria Report (2008) World Health Organization Switzerland,pp: 99-101.
- Agomo CO, Oyibo WA, Anorlu RI, Agomo PU (2009) Prevalence of malaria in pregnant women in Lagos, South-West Nigeria. Korean J Parasitol 47: 179-183.
- Eze NCE, Nzeako SO, Amadi EC (2010) Current status of malaria and urban schistosomiasis infections in the Mammy Market Free Zone of the 34th Field Artillery Brigade in Obinze, Owerri. Nig J of Parasitol 31: 60-68.
- Bennuru S, Nutman TB (2009) Lymphatics in human lymphatic filariasis: in vitro models of parasite-induced lymphatic remodeling. Lymphat Res Biol 7: 215-219.
- Eze NC, Nzeako SO, Nduka FO (2014) Patterns of plasmodium falciparum among settled fulani pastoralists in rivers state, Nigeria. Int JTrop Dis Health 4: 295-305.
- Das D, Kumar S, Dash AP, Babu BV (2005) Knowledge of lymphatic filariasis among the population of an endemic area in rural Madhya Pradesh, India. Ann Trop Med Parasitol 99: 101-104.
- Zeldenryk LM, Gray M, Speare R, Gordon S, Melrose W (2011) The emerging story of disability associated with lymphatic filariasis: a critical review. PLoS Negl Trop Dis 5: e1366.
- Sherman IN (1998) A brief history of malaria and discovery of the parasites life cycle. In: malaria parasite biology, pathogenesis and protection. In: Sherman IN (edn.) ASM Press, Washington DC, USA,pp: 97-108.
- Ebrahim S (2001) Theintolerable burden of malaria: a new look at the numbers. World Health Organization, regional office for Africa Harare, Zimbabwe. Am Soc Trop Med Hyg 64: 1.
- Townson H, Nathan MB, Zaim M, Guillet P, Manga L, et al. (2005) Exploiting the potential of vector control for disease prevention. Bull World Health Organ 83: 942-947.
- Gbakima AA, Appawu MA, Dadzie S, Karikari C, Sackey SO, et al. (2005) Lymphatic filariasis in Ghana: establishing the potential for an urban cycle of transmission. Trop Med Int Health 10: 387-392.
- Terranella A, Eigiege A, Gontor I, Dagwa P, Damishi S, et al. (2006) Urban lymphatic filariasis in central Nigeria. Trop Med Parasitol 100: 163-172.
- Castro MC, Kanamori S, Kannady K, Mkude S, Killeen GF, et al. (2010) The importance of drains for the larval development of lymphatic filariasis and malaria vectors in Dares Salaam, United Republic of Tanzania. PLoS Neg Trop Dis 4:e693.
- Khosa E, Kuonza LR, Kruger P, Maimela E (2013) Towards the elimination of malaria in South Africa: a review of surveillance data in Mutale Municipality, Limpopo Province, 2005 to 2010. Malar J 12: 7.
- Simonsen PE, Mwakitalu ME (2013) Urban lymphatic filariasis. Parasitol Res 112: 35-44.
- World Malaria Day (2016) End malaria for good. Malaria consortium.
- Nigeria Malaria Fact Sheet (2011) Economic sections, United States embassy in Nigeria plot 1075, diplomatic drive central area abuja, FCT, Nigeria.
- Martin PH, Lefebvre MG (1995) Malaria and climate: Sensitivity of malaria potential transmission to climate Ambio24: 200-207.
- Beier JC, Killeen GF, Githure J (1999) Short report: entomologic inoculation rates and plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg61: 109-113.
- Ukpai MO, Ajoku EI (2001) The prevalence of malaria in Okigwe and Owerri areas of Imo State. Niger J Parasitol 22:43-48.
- World Health Organization (1992) Lymphatic filariasis: the disease and its control. Fifth report of the WHO Expert Committee on Filariasis. World Health Organ Tech Rep Ser 821: 1-71.
- Ukaga CN, Nwoke BEB, Onyeka PIK (2003) Integrating women in disease management: case of malaria. Niger J Parasitol 24:53-58.
- Malaria: Know the facts (1998) World Health Organization. Newsletter13:6-7.
- Mbanugo JI, Ejims DO (2000) Plasmodium infections in children Aged 0-5 yrs in Awka metropolis, Anambra State, Nigeria. Niger J Parasitol 21:55-59.
- Aribador DN, Njoku OO, Eneanya CI, Onyali, IO (2003) Studies on prevalence of malaria and management practices of the azia community, in ihiala local government area, Anambra State, South –East Nigeria. Nig J Parasitol 24:33-38.
- Ezeanya CI (1998) Seasonal variation in malaria episodes among residents in Udi, a semi urban community in southeast Nigeria. Nig J Parasitol 19:39-43.
- Oparaocha ET (2003) The impact of heamoglobin level and concomitant infections of malaria parasitaemia and on-set of fever during malaria attack in Ikwuano Local Government Area of Abia State.Nig J Parasitol 24:25-32.
- Annual report on lymphatic filariasis (2002) Geneva, WHO.
- Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, et al. (2003) Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg 68: 169-176.
- Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, et al. (2006) Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol 43: 1215-1221.
- Wickramage K, Premaratne RG, Peiris SL, Mosca D (2013) High attack rate for malaria through irregular migration routes to a country on verge of elimination. Malar J 12: 276.
- Michael E, Bundy DA (1997) Global mapping of lymphatic filariasis. Parasitol Today 13: 472-476.
- World Bank, Africa Regional Office (2009) Intensifying the fight against malaria: The World Bank booster program for malaria control in Africa. World Bank Publications, Washington, DC, USA.
- Amadi EC, Udonsi JK (2004) Studies on the influence of climatic factors on the transmission of potential mosquito vectors of lymphatic filariasis in Khana L.G.A of rivers state.Nig J Parasitol ,p: 90.
- Ojiako O, Onyeze G (2008) Epidemiological and biochemical studies of human lymphatic filariasis and associated parasitoses in oguta, southeasternNigeria. Internet J Parasit Dis 4: 1.
- Okorie PN, Ademowo GO, Saka Y, Davies E, Okoronkwo C, et al.(2013) Lymphatic filariasis in Nigeria; micro-stratification overlap mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl Trop Dis 7: e2416.
- Michael E, Bundy DAP, Grenfell BT (1996) Re-accessing the global prevalence and distribution of lymphatic filariasis. Bulletin of the World Health Organisation75:491-503.
- Eigege A, Richards FO, Blaney DD, Miri ES, Gontor I, et al. (2001) Rapid assessment for lymphatic filariasis in central nigeria: a comparism of the ICT and hydrocoete rates in an area of high endemic. Am J Trop Med Hyg68:643-646.
- World Malaria Situation in 1994 (1997) Weekly epidermiological record. WHO72:269-276.
- https://www.news-medical.net/?tag=/Elephantiasis&page=2
- Anosike JC, Onwuliri COE (1992) Experimental Wuchereriabancrofti infection in Culexquinquefasciatus and Aedesaegypti. Angew Parasitol 33:139-141.
- Anosike JC, Nwoke BEB, Onwuliri COE, Dozie INS (2003) Laboratory Investigation of the infection rates Anopheles gambiae and An. funestus in the transmission of W. bancrofti. Niger J Parasitol 24:143-148.
- Mbah DE, Njoku OO (2004) Vectors of lymphatic filariasis in anambra state south eastern, Nigeria.Niger J Parasitol , p: 57.
- Amaechi AA (2009) Studies on insecticide impregnated bed nets for control of mosquito vectors of lymphatic filariasis in parts of Ebonyi State, Nigerian. Ph.D. Thesis of Imo State University Owerri, Nigeria.
- Nwoke BEB, Dozie INS, Jiya J, Saka Y, Ogidi JA, et al. (2006) The prevalence of hydrocoele in Nigeria, and its complication on mapping of lymphatic filariasis. Niger J Parasitol 27: 29-35.
- www.info.com/Maps+Of+Rivers
- Omudu EA, Okafor FC (2011) Study of chronic lymphatic filariasis related knowledge, attitude and perception among three ethnic groups in Benue State, Nigeria. Niger J Parasitol 32:135-142.
- Okeoguale B, Dadiyel JA (2014) Nigeria launches Africa’s first nationwide malaria lymphatic filariasis (elephantiasis) elimination. a report: neglected tropical diseases network:A global forum, non-governmental organization working together on NTD’s.
- Nazeh MA, Zurianee MN, Abdulhamid AAH, Al-addhroey M, Mustafa K (2014) Lymphatic filariasis in peninsular Malaysia: a cross sectional survey of the knowledge, attitude and practice of residents. Parasit Vectors 7: 545.
- Bandyopadhyay L (1996) Lymphatic filariasis and the women of India. SocSci Med 42: 1401-1410.
- WHO (2002) Roll back malaria. World Health Organization Fact, sheet No.203. Geneva, Switzerland.
- Roll back malaria progress and impact series (2011) Business investing in malaria control: economic return and a health workforce for Africa. WHO, Geneva.
- https://www.population.gov.ng/index.php/censuses.
- Nzeako SO, Nduka FO, Origie OA (2013) Prevalence of malaria in pregnant women attending ante natal care at university of port harcourt primary health care centre aluu, port harcourt, rivers state. Niger Int J Scient Res Environ Sci 1: 268-272.
- Gyapong M, Gyapong JO, Adjei S, Vlassof C, Weiss M (1996) Filariasis in Northern Ghana: Some cultural beliefs and practices and their implications for diseases control. SocSci Med 43:235-242.
- Babu BV, Nayak AN, Dhal K, Acharya AS, Jangid PK, et al. (2002) The economic loss due to treatment costs and work loss to individuals with chronic lymphatic filariasis in rural communities of Orissa, India. Acta Trop 82: 31-38.