- (2013) Volume 14, Issue 4
Chakra P Chaulagain1, Janice Rothschild2, Muhammad Wasif Saif1
1Division of Hematology and Oncology and Experimental Therapeutics and 2Department of Surgery, Tufts Medical Center and Tufts University School of Medicine. Boston, MA, USA
There remains a lack of consensus on the optimal adjuvant therapy for pancreatic cancer. In general, chemoradiation is favored in the United States and gemcitabine based chemotherapy is favored in Europe. Both of these approaches have been shown by large prospective, randomized trials to improve disease free survivals and in some studies overall survival. We present the summary of three abstracts from the 2013 American Society of Clinical Oncology (ASCO) Annual Meeting and discuss their potential impact on our clinical practice. Adjuvant oral chemotherapy with S-1 (Fukutomi et al., Abstract#4008) has now emerged as a promising alternative to the traditional gold standard of intravenous gemcitabine in a relatively large randomized phase III clinical trial. Another study by Yoshitomi et al. (Abstract #4056) examined the value of adjuvant chemotherapy with S-1 alone versus combination of S-1 and gemcitabine versus gemcitabine alone in a three arm phase II clinical trial (CAP-002 Study). In terms of biomarkers in pancreatic cancer, Neoptolemos et al. presented the impact of hENT1 tumor levels on the outcome of the patients with pancreatic cancer (Abstract #4006) who had received adjuvant chemotherapy with either 5-flurouracil or gemcitabine in the ESPAC trial.
Biological Markers; Chemotherapy, Adjuvant; Equilibrative Nucleoside Transporter 1; Pancreatic Neoplasms; S 1 (combination)
CDHP: 5-chloro-2,4-dihydroxypyridine; CONKO: Charité Onkologie; ESMO: European Society of Medical Oncology; ESPAC: European Study Group for Pancreatic Cancer; GEST: Gemcitabine and S-1 Trial Group; hENT: human equilibrative nucleoside transporter; HR: hazard ratio; JASPAC: Japanese Adjuvant Study Group of Pancreatic Cancer; NCCN: National Comprehensive Cancer Network; RTOG: Radiation Therapy Oncology Group
Clinical trials for utilizing adjuvant chemotherapy in pancreatic cancer are mostly from Europe. The European Study Group for Pancreatic Cancer (ESPAC-1) trial in 2001 examined the value of chemoradiation and chemotherapy in a 2x2 fractional design. The chemotherapy (5-flurouracil and leucovorin) versus observation arms showed an overall median survival of 20.1 months versus 15.5 months (P=0.009) [1]. Another phase III randomized German trial, Charité Onkologie Clinical (CONKO)-001, in 2007 compared adjuvant gemcitabine versus observation. Gemcitabine showed superiority over observation with the median disease free survival of 13.4 months versus 6.9 months (P<0.001) and median overall survival of 22.8 months versus 20.2 months (P=0.005) [2, 3]. The ESPAC-3 trial in 2009 compared gemcitabine versus bolus 5-flurouracil plus leucovorin showing a non-significant difference in median overall survival of 23.6 months versus 23 months [4]. Though the survival was similar in both gemcitabine and 5- flurouracil group, there were greater toxicities with 5-flurouracil based chemotherapy. This is why gemcitabine is considered the preferred adjuvant chemotherapeutic agent.
The Radiation Therapy Oncology Group (RTOG) 9704 trial (July 1998-July 2002) randomized patients after gross total resection of pancreatic cancer to receive either 5-flurouracil or gemcitabine for 3 weeks prior to chemoradiation therapy and for 12 weeks after chemoradiation therapy. Chemoradiation with a continuous infusion of 5-flurouracil was the same for all patients [5]. There was no difference in the two groups except for patients with pancreatic head tumors where a non-statistically significant improvement in survival was noted with gemcitabine group; i.e., the median survival was 20.5 months in gemcitabine group versus 16.9 months in the 5-flurouracil group (P=0.09). Though not a robust finding, this trial helped to establish the value of adjuvant gemcitabine-based chemotherapy along with 5- flurouracil-based concurrent chemoradiation. This approach currently represents the most common clinical practice in the USA.
The quest for optimal adjuvant therapy continues and the use of oral agent S-1 in adjuvant setting represents one of the newer successes in this direction. In general, both National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO) encourage enrolling patients to clinical trials evaluating potential benefits of chemotherapy or combined modality therapy with chemoradiation.
Pancreatic cancer being a highly lethal disease without effective therapy remains a fertile ground for testing new treatment modalities with only limited success. Certainly, more innovative treatment approaches are urgently sought to improve survival in this patient population. This year in the ASCO Annual Meeting, Fukutomi et al. (Abstract #4008) presented the results of phase III randomized trial of adjuvant chemotherapy with gemcitabine versus S-1 for patients with resected pancreatic cancer (Japanese Adjuvant Study Group of Pancreatic Cancer; JASPAC-01 study) [6, 7, 8]. The study conducted in 33 centers in Japan showed that S-1 is non-inferior and may be superior with tolerable side effect profile. S-1 has now emerged as a potential first line alternative to gemcitabine in adjuvant setting for Japanese patients. The results of the phase II triple arm trial comparing S-1 alone, combination of S-1 and gemcitabine and gemcitabine alone (Abstract #4056) [9] has now set up an avenue for studying this approach in a larger phase III trial. The findings of the retrospective biomarker study on ESPAC trials, supports the use of gemcitabine in patients with high tumor human equilibrative nucleoside transporter 1 (hENT1) expression and 5-flurouracil in patients with low hENT1 (Abstract #4006) [10].
S-1 (Taiho Pharmaceutical, Tokyo, Japan) is an oral fluoropyrimidine derivative shown to be effective for gastric and various other types of cancers. It is designed with the aim of improving antitumor activity and reducing the toxicity of 5- fluorouracil. This novel molecule consists of tegafur, a prodrug of 5-flurouracil combined with two 5- flurouracil biochemical modulators: 5-chloro-2,4- dihydroxypyridine (gimeracil or CDHP), a competitive inhibitor of dihydropyrimidine dehydrogenase and oteracil potassium which inhibits phosphorylation of 5-flurouracil in the gastrointestinal tract thereby decreasing serious gastrointestinal toxicities such as nausea, vomiting, stomatitis and diarrhea [8, 11] (Figure 1). This oral agent offers several advantages over 5-flurouracil including ease of administration, less toxicities and no risks associated with use of central venous access such as infection, thrombosis and bleeding.
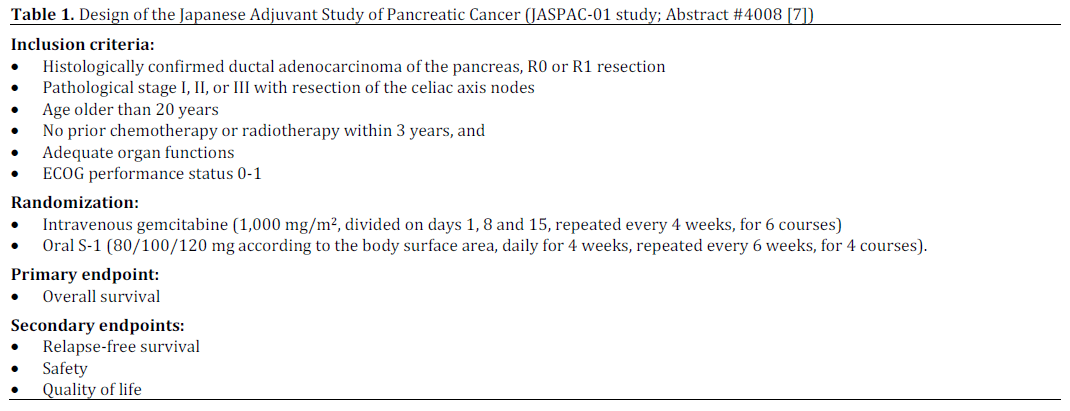
Randomized Phase III Trial of Adjuvant Chemotherapy with Gemcitabine Versus S-1 for Patients with Resected Pancreatic Cancer (JASPAC-01 Study; Abstract #4008 [7])
The aim of this phase III study was to determine non-inferiority of S-1 to gemcitabine on overall survival as adjuvant chemotherapy for resected pancreatic cancer. This study enrolled 385 patients (gemcitabine, n=191; S-1, n=187) between April 2007 and June 2010. The primary end point was overall survival. Secondary end points were relapse-free survival, safety and quality of life. The reported 2-year overall survival rates were 53% for gemcitabine and 70% for S-1 favoring S-1 with hazard ratio of 0.56 (95% CI: 0.42-0.74; P<0.0001 for non-inferiority, and P<0.0001 for superiority). The details are presented in Tables 1, 2, 3 and 4.
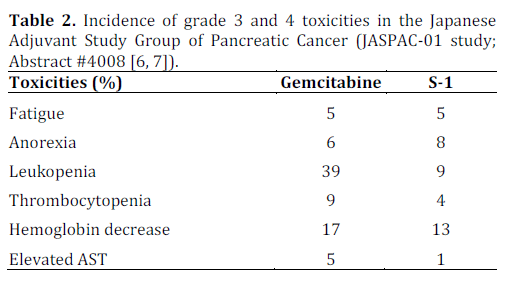
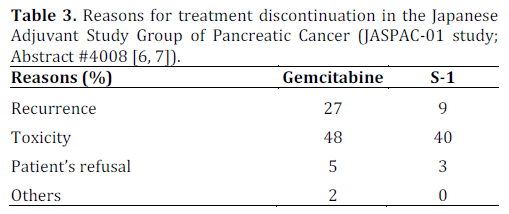
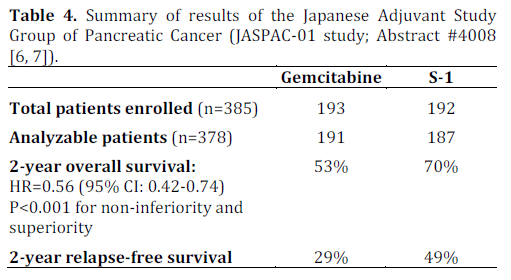
This multicenter phase III study from Japan in patients with pancreatic cancer clearly showed noninferiority and even superiority of S-1 to gemcitabine in the adjuvant setting. Though the trial was designed to show non-inferiority only, the results were found to show superiority as well. The grade 3 and 4 toxicities were comparable in both arms with less myelosupression in patients receiving S-1. In addition, the quality of life data were in favor of S-1 compared to gemcitabine (P<0.0001). The results are potentially practice changing as the findings have challenged the long standing traditional gold standard of gemcitabine as the choice of adjuvant chemotherapy. The result is also likely to influence the design of future clinical trials in Asia and beyond as similar trials assessing the utility of S-1 in adjuvant setting are likely to be designed for European and North American population. A longer term follow up (e.g., 5 years) is warranted to see if the advantage of S-1 to gemcitabine lasts beyond 2-year time point and translates into long term survival. Pancreatic cancer experts throughout the world are currently waiting to see the final publication of this study which will shed more lights into the details on study design, setting, participants, study methodology used, outcome measures, any potential confounding variables and their relevance to patients with pancreatic cancer.
The ESPAC-3 study showed similar survival outcome between 5-flurouracil versus gemcitabine in the adjuvant setting though the safety and dose intensity favored gemcitabine. This study, however, helped to bring back 5-flurouracil and other fluoropyrimidines such as capecitabine and S-1 on the stage for further assessment in clinical trials. While discussing applicability of S-1 in the North American population, it is important to recollect that multinational clinical studies of another oral fluoropyrimidine capecitabine in gastrointestinal cancers has shown significantly worse toxic-effect profile (mainly diarrhea) in patients recruited from the United States compared to those from Asia. These early clinical studies of S-1 in the USA showed diarrhea as the dose-limiting toxicity whereas the Japanese studies showed myelosuppression as the dose-limiting toxicity. This differential side effects and tolerability profile between populations is probably due to polymorphisms in the CYP2A6 gene [12] that encodes CYP2A6 the principal enzyme responsible for bioconversion of tegafur to 5- flurouracil. One important question to be addressed in the future clinical trials is whether or not a reduced dose of S-1 will cause less severe diarrhea while retaining therapeutic efficacy in pancreatic cancer patients. At this point, gemcitabine remains the agent of choice both in Europe and North America as adjuvant chemotherapy in resected pancreatic cancer.
Randomized Phase II Trial of Adjuvant Chemotherapy with S-1 Versus S-1 and Gemcitabine versus Gemcitabine Alone in Patients with Resected Pancreatic Cancer (CAP-002 Study) (Abstract #4056 [9])
The phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan (Gemcitabine and S-1 Trial Group; GEST Study) showed that monotherapy with S-1 was noninferior to gemcitabine in prolonging overall survival [13] (S-1 versus gemcitabine: HR=0.96 (97.5%CI: 0.78-1.18; P<0.001 for non-inferiority). The combination of gemcitabine and S-1 showed no overall survival advantage but showed longer progression free survival in the combination group compared to gemcitabine alone (gemcitabine and S- 1 versus gemcitabine alone: HR=0.66; P<0.001 for superiority) [13]. The lack of overall survival with the combination could be because of the fact that the patients who progressed on gemictabine went on to receive S-1 as second line therapy. The JASPAC 01 study showed non-inferiority of S-1 compared to gemcitabine in the adjuvant setting. Both of these studies also highlighted the advantages of S-1 including better tolerability and convenience of oral administration. This is the background rationale behind this phase II trial comparing the combination therapy with S-1 and gemcitabine to single agent gemcitabine or single agent S-1. The findings are summarized in Table 5.
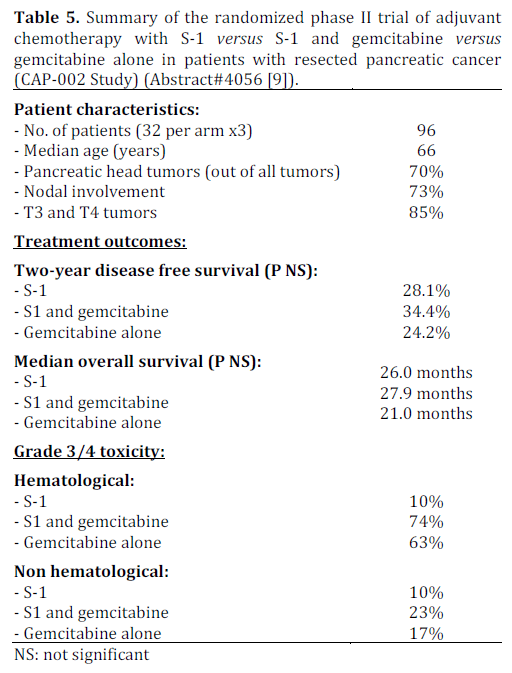
This phase II trial did not show any significant survival advantage for the combination of gemcitabine and S-1 compared to either agent alone with trend towards slightly higher toxicities with the combination. The JASPAC 01 trial has shown that S-1 is non-inferior to gemcitabine as adjuvant chemotherapy but it is unknown if the combination of S-1 and gemcitabine provides additional benefits with the cost of added toxicities as the combination was found to cause significantly higher grade 3 or greater myelosupression, rash and mucositis in GEST study [13]. Therefore, this approach deserves further testing in a phase III trial in adjuvant setting.
hENT1 Tumor Levels to Predict Survival of Pancreatic Ductal Adenocarcinoma Patients Who Received Adjuvant Gemcitabine and Adjuvant 5- Flurouracil on the ESPAC Trials (Abstract #4006 [10])
Using microarray techniques, Neoptolemos et al. studied expression levels of human equilibrative nucleoside transporter (hENT1) and its correlation with the response to chemotherapy with 5- flurouracil (n=176) and gemcitabine (n=176) using tumor specimens from patients in the ESPAC-1 and 3 trials [10]. The results are shown in Table 6.
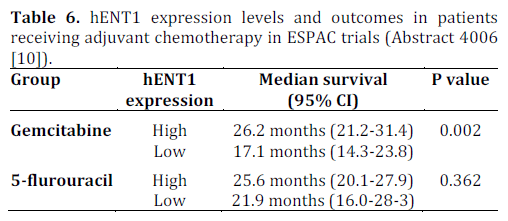
This retrospective study showed that higher expression of hENT1 was associated with greater response to adjuvant gemcitabine therapy (P=0.002). As expected, there was no correlation between the levels of hENT1 expression and response to 5-flurouracil. The protein hENT1 is a membrane transporter required for intracellular uptake of gemcitabine and is expressed by pancreatic cancer cells at varying levels. The drug CO-101 (CP-4126, Clovis Oncology, Boulder, CO, USA) is a lipid-conjugate of gemcitabine designed to enter the pancreatic cancer cell independent of hENT1 thereby circumventing the primary resistance due to inadequate expression of hENT1. This drug was tested in randomized phase II clinical trial (n=367) with the intent to show that CO-101 improves overall survival versus gemcitabine in patients with untreated metastatic pancreatic adenocarcinoma whose tumors have a low level of hENT1 [14]. The study also examined on a prospective basis, if higher hENT1 expression was associated with better outcome in patients receiving gemcitabine. The results were essentially negative concluding that the tumor levels of hENT1 do not predict response to treatment with gemcitabine or CO-101 [14]. This was also evaluated in neoadjuvant setting in a study from Japan (n=63) that did not find an association between expression level of hENT1 and outcome for pancreatic cancer patients treated with neoadjuvant chemoradiation that contained gemcitabine as chemotherapy [15]. In summary, the results of studies on hENT1 expression on outcomes are conflicting as the current study in the adjuvant setting showed correlation of high hENT1 expression and response to gemcitabine and the other studies did not show such correlation in the metastatic and neoadjuvant setting. This discrepancy may be due to potential difference in the level of expression of hENT1 (quantitative) or its function (qualitative) as the pancreatic cancer evolves from organ confined disease to a metastatic cancer. Prospective studies of hENT1 expression in adjuvant setting are desirable and, until such studies are done, hENT1 expression as a marker will remain limited in clinical trial.
Dr. Chakra Chaulagain’s work in this article is supported by translational research fellowship (unrestricted educational grant) for academic year 2012/2013 from Genentech BioOncology, Inc., San Francisco, CA, USA
None