- (2013) Volume 14, Issue 5
Stephanie LM Das1, George I Papachristou2, Tercio De Campos3, Jozefa Panek4, Ignasi Poves Prim5, Alejandro Serrablo6, Rowan W Parks7, Generoso Uomo8, John A Windsor1, Maxim S Petrov1, on behalf of the Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA)
1Department of Surgery, The University of Auckland. Auckland, New Zealand. 2Division of Gastroenterology, Hepatology and Nutrition, Department of Medicine, University of Pittsburgh Medical Center. Pittsburgh, PA, USA. 3Department of Surgery, “Santa Casa” School of Medical Sciences. São Paulo, Brazil. 4Second Department of General Surgery, Jagiellonian University Medical College. Cracow, Poland. 5Unit of Hepato-Biliary-Pancreatic Surgery, Service of General and Digestive Surgery, Hospital del Mar. Barcelona, Spain. 6HPB Surgical Unit, Miguel Servet University Hospital. Zaragoza, Spain. 7Department of Clinical and Surgical Sciences (Surgery), University of Edinburgh, Royal Infirmary of Edinburgh. Edinburgh, United Kingdom.
8Department of Internal Medicine, Cardarelli Hospital. Naples, Italy
Context Organ failure is a major determinant of mortality in patients with acute pancreatitis. These patients usually requireadmission to high dependency or intensive care units and consume considerable health care resources. Given a low incidence rate of organ failure and a lack of large non-interventional studies in the field of acute pancreatitis, the characteristics of organ failure that influence outcomes of patients with acute pancreatitis remain largely unknown. Therefore, the Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA) aims to conduct a meta-analysis of individual patient data from prospective non-interventional studies to determine the influence of timing, duration, sequence, and combination of different organ failures on mortality in patients with acute pancreatitis. Methods Pancreatologists currently active with acute pancreatitis clinical research will be invited to contribute. To be eligible for inclusion patients will have to meet the criteria of acute pancreatitis, develop at least one organ failure during the first week of hospitalization, and not be enrolled into an intervention study. Raw data will then be collated and checked. Individual patient data analysis based on a logistic regression model with adjustment for confounding variables will be done. For all analyses, corresponding 95% confidence intervals and P values will be reported. Conclusion This collaborative individual patient data meta-analysis will answer important clinical questions regarding patients with acute pancreatitis that develop organ failure. Information derived from this study will be used to optimize routine clinical management and improve care strategies. It can also help validate outcome definitions, allow comparability of results and form a more accurate basis for patient allocation in further clinical studies.
Meta-Analysis as Topic; Mortality; Multiple Organ Failure; Organ Dysfunction Scores; Pancreatitis, Acute Necrotizing /complications
IPD: individual patient data; SOFA: Sepsisrelated Organ Failure Assessment
Organ failure is a major determinant of mortality [1, 2, 3, 4, 5] in patients with acute pancreatitis and this has been highlighted in the new international multidisciplinary classification of acute pancreatitis severity as the “systemic” determinant [1, 2]. Patients who develop organ failure usually require admission to high dependency unit or intensive care unit (ICU). These patients are among the most resource demanding in health care systems [6, 7]. ICUs currently represent the largest clinical cost department in hospitals, with expenses estimated to be up to 20% of a hospital’s budget [8, 9], and costs per day is three to five-fold greater than in general wards [10, 11, 12].
Several aspects of organ failure have been studied, although many questions remain.
1) Dynamics of Organ Failure
It is widely accepted that local pancreatic inflammation is the initiating stimulus for a systemic inflammatory response. This, in turn, may result in the development of organ failure and contribute to death in patients with acute pancreatitis. The importance of the duration and reversibility of organ failure has been well recognized in the last decade. It has been shown that patients with worsening organ failure as well as those with persistent organ failure have a significantly higher mortality rate [13, 14, 15, 16, 17]. A study from the United Kingdom [14] enrolled 290 patients across 18 centers and found that resolution of organ failure within 48 hours was associated with a better prognosis compared to patients with organ failure for more than 48 hours. This was irrespective of whether the organ failure was present on admission or developed later. Another study conducted in Scotland [13] on 121 patients found that patients with worsening organ failure and poor response to treatment have a higher mortality rate. A study from New Zealand also demonstrated that the initial physiological response to intensive care treatment was a better predictor of outcome and mortality in patients with acute pancreatitis [17]. These studies underscore the dynamic nature of organ failure and the importance of monitoring the response to treatment.
2) Number of Failed Organs
The concept of multiple organ failure was first described in the 1970s [18], since then, a large number of definitions and acronyms have been proposed [19]. A modern definition for multiple organ failure in patients with acute pancreatitis refers to the failure of two or more organ systems [20]. Multiple organ failure has been shown to be the leading cause of death in a variety of clinical settings [21, 22]. Mortality rate in acute pancreatitis patients with multiple organ failure has been reported to be up to 100% [20, 23, 24, 25, 26] and there is a significant correlation between the number of organ failures and mortality [27]. A large population based retrospective cohort study on all deaths due to acute pancreatitis in Scotland included data from 1,024 patients and found that 63% of fatalities had failure of at least two organ systems [28].
3) Which Organs Fail
The Atlanta classification for severity of acute pancreatitis advocated four organ systems (cardiovascular, pulmonary, renal and gastrointestinal bleeding) to classify the severity of acute pancreatitis [22]. The European Society of Intensive Care Medicine since advocated a scoring system that includes six major organ systems to describe as quantitatively and objectively as possible the degree of organ dysfunction over time in critically ill patients [29]. This scoring system, termed the Sepsis-related Organ Failure Assessment (SOFA) score, has been widely used in a variety of disease settings [30, 31, 32]. The organ systems used in the SOFA score are respiratory, cardiovascular, coagulation, hepatic, renal and central nervous systems. The number of organ systems that have been included in studies have varied from three [4], four [27, 33], six [34, 35] to eight [27]. Based on the 2011 global survey of pancreatologists [36], the consensus is that three organ systems (respiratory, cardiovascular and renal) fail most frequently in patients with acute pancreatitis and is of much more prognostic importance than failure of other systems [4, 35, 37, 38]. Presence/absence of organ failure in each of these three systems is used in the new international multidisciplinary classification of acute pancreatitis severity [1].
4) Combination and Sequence of Organ Failures
A prospective multicenter inception cohort analysis [39] of 17,440 ICU admissions (all cases and not confined to patients with acute pancreatitis) treated from 1988 to 1990 and 5,677 ICU admissions treated from 1979 to 1982 found that combinations of organ systems and the organ system that failed had an impact on outcome. They found that the profile of physiologic abnormalities substantially influences mortality. For example, mortality rate for patients with two organ system failures varied from 20% (combination of hematologic and cardiovascular failure) to 76% (combination of cardiovascular and neurologic failures).
In patients with acute pancreatitis, a retrospective study by Halonen et al. [35] demonstrated that different combinations of two organ system failures have different mortality rates with the highest mortality rate (91%) associated with the combination of hepatic and renal failures. They also showed that hepatic failure, renal failure, previous cardiovascular medication and cardiovascular failure were independent factors that are associated with hospital mortality. Some limitations of this study include the retrospective design, the relatively small cohort of patients (n=113) and a selection bias because not all patients with organ failure were included.
Another study looked at sequential system failure in patients with acute renal failure after rupture of abdominal aortic aneurysms [40]. The authors showed that there was a similar progression of organ system failures in all patients. This sequence unfolded more slowly in patients that survived longer and developed more quickly in those surviving for shorter periods. This “predictability” of sequence failure in organ failure, if confirmed, may have important implications in the allocation of resource and targeted treatments directed towards slowing disease progression and reducing mortality.
Limitations of Current Knowledge About Organ Failure
First, most studies have been single center cohort studies [15, 16, 41, 42, 43, 44] without sufficient statistical power to investigate all aspects of organ failure and their effect on mortality. This is likely because of relatively low incidence of acute pancreatitis patients with organ failure. The annual incidence rate in the United States is 2-4 cases of complicated acute pancreatitis per year per 100,000 adults, and only a fraction of them develop organ failure [45].
Second, there are some studies in which the cohorts from larger multi-center studies are part of interventional studies [14, 46] which makes any inference about disease course and outcomes in general, and organ failure in particular, inherently biased. This is because any studied intervention is designed to alter the natural course of the disease. Other larger studies are limited by selection bias, such as using preselected cohorts of patients with other determinants of outcome (e.g., pancreatic necrosis) or only selected patients who had a contrast enhanced CT scan [33, 47, 48, 49]. This selection bias does not allow a valid inference about the course and outcomes of organ failure.
Third, there is a relatively limited body of evidence in the literature about the relative importance of different characteristics of organ failure such as number, timing, duration, sequence, and combination [1]. Moreover, there is limited evidence to validate the definitions of these characteristics. Valid outcome definitions are essential for quality research, allowing comparability of results among centers and the ability to monitor changes in between different centers over time [50].
Unanswered Questions About Organ Failure
Limitations and bias in the existing literature highlights the need for purportedly designed noninterventional studies to answer a number of key questions relating to the characteristics of organ failure in acute pancreatitis [51] and how they are linked to mortality (Figure 1). These include, but are not limited to the following questions:
1) What is the relative incidence of each organ failure?
2) What is the relationship between number of organ failures and mortality?
3) What is the most common sequence in failing systems?
4) What is the timing of onset for each organ failure and its effect on mortality?
5) How is mortality affected by the duration of each system failure?
6) What is the relative incidence of the specific sequences of organ failure and its effect on mortality?
7) What is the relative incidence of each combination of two system failures and its effect on mortality?
Answering the Questions by Conducting an Individual Patient Data Meta-Analysis
Pooled analysis of prospective data from individual patients in all the available studies has been regarded as the gold standard in evidence synthesis generation [52, 53] and has provided the best approach to answer questions pertinent to the natural course of disease [54]. The methods and advantages of individual patient data (IPD) metaanalysis have been well described [55, 56]. IPD meta-analysis provides the least biased and most reliable means of addressing questions not satisfactorily answered by individual clinical studies [57]. This is because it does not rely on published information alone and includes all available study data, thus allowing for detailed checks of the integrity and completeness of data and also reducing selection and publication bias. By including data from multiple centers, it provides a stronger endorsement of results, better clarification and provision of updated follow up information, as well as a collaboration for further research [58]. In addition to this, it allows for more powerful and flexible analysis of subgroups and testing, adjusting for confounders.
The aim of the proposed study is to perform an IPD meta-analysis to determine the relative incidence of each organ failure, and the impact that the number, timing, duration, sequence, and combination of different individual organ failures on mortality in patients with acute pancreatitis.
Study Design
The study design will be an individual patient data meta-analysis [55, 56, 57, 58].
Identification of Studies
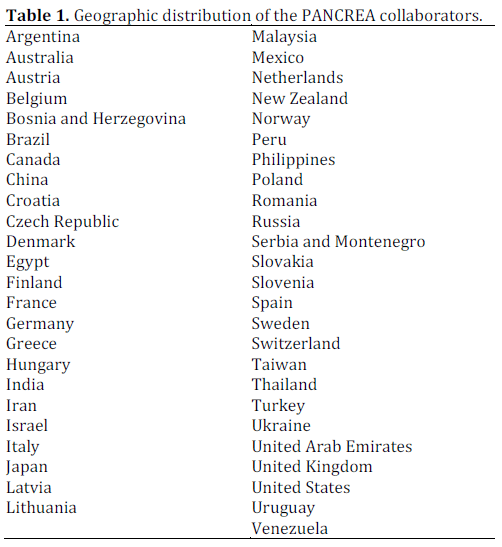
All participants of the recently conducted first global survey of pancreatologists, who are active in clinical research, will be invited to contribute individual patient data to this study [36]. Table 1 presents the geographic distribution of participants in the global survey. Pancreatologists are also encouraged to contact the corresponding author of this article if they are interested in contributing to this project.
Eligibility Criteria
To be included, studies will have to meet the following criteria:
• Design: prospective cohort;
• Population: patients with acute pancreatitis who presented with or developed organ failure during first week of hospital admission;
• Exposure: respiratory, renal, and/or cardiovascular organ failure;
• Outcome: in-hospital mortality;
• Study period: conducted from the year 2000 onwards.
Studies/individual data will be excluded if:
• Participants were enrolled into an interventional study;
• Data do not contain the essential information required (see below).
Collection of Data and Management
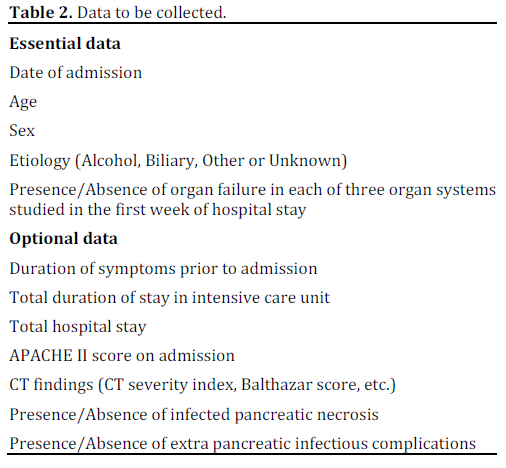
Essential and optional data to be collected are shown in Table 2. All the contributors will be asked to provide de-identified data by uploading them into a standardized data collection form or in any convenient format by encrypted, electronic transfer where possible or by other means as required, depending on site issues. The original data collection files sent by the authors will be kept in their original version and will be saved on a password-protected server at the University of Auckland, and behind the firewall to ensure security. Only the investigators of PANCREA II study will have direct access to individual data prior to publication of the final report.
Transfer of Data
The data will be transferred to a secure passwordprotected web server at University of Auckland or by privacy encrypted e-mail. This permits a secure and identifiable connection and minimizes the possibility of data loss.
Data Checking
Study investigators will perform data validation using a copy. The data will be checked independently with respect to range, internal consistency, missing or extreme values, errors and consistency with published reports. Study details, such as selection methods and outcome details will be crossed-checked against published reports, study protocols and data collection spread sheets. Apparent inconsistencies, implausibilities, or omissions will be clarified with collaborators and, where appropriate, rectified. Summary tables and listing of the variables used in planned analyses will be supplied to collaborators for checking. Any discrepancies will be resolved by discussion. Collaborators will be asked to verify all recorded data before any analysis and the data will not be used for any other purposes without permission from all the collaborators.
Core Data Set and Variables
All verified data will be entered into a master Excel spread sheet. A unique identification number will be allocated to each patient entered into the core data set. This number will easily correspond to patients from verified data from individual studies. The essential and optional data will be manually entered into master spread sheet, and checked.
Acute pancreatitis will be diagnosed by the presence two of the following three features:
• abdominal pain characteristic of acute pancreatitis;
• serum amylase and/or lipase 3 times the upper limit of normal; and
• characteristic findings of acute pancreatitis on tomography (CT) scan.
Organ failure will be defined as the presence of worsened organ function in an acutely ill acute pancreatitis patient using one of the following criteria:
• “breaching of thresholds” as described by Bradley et al. [22] with shock defined as a systolic blood pressure less than 90 mmHg, pulmonary insufficiency defined as PaO2 of 60 mmHg or less, renal failure defined as a creatinine level greater than 177 μmol/L (2 mg/dL) after rehydration;
• a SOFA score of 2 or more for each individual system [29];
• a Marshall score of 2 or more for each individual system [59].
The organ systems that will be reported on include respiratory, cardiovascular and renal systems. The definitions used in relation to the timing, duration, sequence and combination of organ failures are given in Figure 2.
Total hospital stay will be defined as the number of consecutive days the patient was in hospital.
Duration of symptoms will be defined as the number of consecutive full days (24 hours) the patient had symptoms before the day of admission, excluding the day of admission.
Planned Statistical Analysis
Due to the complexity of the statistical analyses, the following section represents the planned principal analyses; some modifications and secondary analyses are likely to emerge during the project. However, a detailed statistical analysis plan will be produced before the analysis. Any analysis conducted will be based on the checked and updated IPD from all available studies.
Primary Analysis
A “one-stage” approach will be used because of its increased power and ability to test for nonlinear relationships for continuous variables and ability to control for aggregation bias [53, 58, 60, 61, 62]. The model used will be based on a logistic regression model [52] adjusted for confounding variables including age, sex, etiology, etc. The dependent variable will be mortality and independent variables will initially include the characteristics of organ failure (timing, duration, sequence, and combination of organ failures). The R 2.15.2 framework (R Foundation for Statistical Computing, Vienna, Austria) will be used for statistical analysis [63].
Summary statistics with corresponding 95% confidence intervals (CIs) will be calculated. This will include the pooled incidence of each system failure. Patients will be grouped according to total number of organ failures at any point and pooled incidence for one, two, three organ failures will be calculated with corresponding mortality rates.
Patients will also be grouped according to timing of first (any) organ failure and mortality rates and relative risks will be calculated according to organ failure occurring at any particular day during the first week. Further analysis will be performed based on duration of organ failure. Relative risks of death will be calculated for patients with organ failure for one day only compared to organ failure for more than one day. The same analysis will be done for organ failure for two days compared to organ failure for more than two days and so on (three and four days).
Patients with two or three organ failures with different sequences of organ failures will be grouped together and the pooled incidence of each sequence of organ failure will be calculated.
Lastly, patients will be grouped according to different combinations of two organ failure and relative risks of death will be calculated. These combinations will include cardiovascular and respiratory, cardiovascular and renal, respiratory and renal systems.
Subgroup Analysis
The cohorts will be grouped according to the provision of optional data. These will be listed firstly in a summary table with the following headings: study title, number of patients, data available (yes/no) for covariates: durations of symptoms, APACHE II score on admission, extent of necrosis on CT, CT severity index, infectious pancreatic complications during hospitalization, other infectious complications during hospitalization, as well as data available for secondary outcomes: duration of ICU stay and total hospital stay. If sufficient patient numbers are available, analysis will then be conducted for each covariate in separate subgroups. The same model used in the primary analysis will also be applied for secondary outcomes if sufficient data are available.
Secondary Analysis
Further analyses may include possible confounding factors for the entire patient population identified from our subgroups analysis. Subsequent analysis from the primary analysis will adjust for any additional confounders using multivariate regression to give estimates that are more relevant to individual patients.
Data Presentation
Baseline characteristics of patients will be presented for individual cohorts as well as overall summary statistics. Continuous variables will be presented as mean and standard deviation (or median and range if not normally distributed). Binary and categorical outcomes will be presented as frequency and percentages. We will also report mortality rates both before and after adjustments for confounders.
For all primary and secondary analyses, adjusted risk ratios and corresponding 95% CIs will be presented, along with the corresponding P values. P values less than 0.05 will be regarded as statistically significant. The final meta-analysis will be reported based on relevant guidelines [64, 65].
Publication Policy
The main results of this project will be published and presented under the auspices of the Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA). Up to two researchers from each contributing centre and the PANCREA Steering Committee will be invited to author the manuscript. Results from further papers using the same data set will not be published without approval from all collaborators and will acknowledge the PANCREA collaboration as the source of the data. The PANCREA collaboration will disseminate the findings of its research widely at academic conferences and in journal publications.
Organ failure is one of main causes of death in patients with acute pancreatitis but, to date, there has been a lack of quality data on its natural course and characteristics that influence patients’ outcomes. Part of the drive to improve patients’ outcomes will require a better understanding of the different characteristics of organ failure. The best way to advance this is to aggregate existing prospective data from non-interventional studies under the auspice of an international collaboration. This approach allows for more powerful and flexible analysis of subgroups and testing, adjusting for confounders and minimizes publication and reporting bias [66] and has been described as the “gold standard” of evidence synthesis [55, 56, 57].
The PANCREA collaboration has already been established and its first study was to develop a new classification for the severity of acute pancreatitis [1]. This involved several stages. The first stage was an evidence review to recognize a need for a new classification for the severity of acute pancreatitis and to highlight the limitations of previous classifications. The second stage was conducting a world-wide survey of pancreatologists. The third stage was to further discuss the proposed classification and seek accord on definitions at an international symposium during the 2011 Meeting of the International Association of Pancreatology (Kochi, India). The final document was published as a feature article in the world’s premier surgical journal and was accompanied by a supportive editorial by the author of the Atlanta classification [67]. The new classification has also become available in several languages other than English [68, 69, 70, 71].
The study described in this protocol will be the second multicentre study of the PANCREA collaborative (PANCREA II study). It will attempt to answer important questions regarding the effect of timing, duration, sequence and combination of individual organ system failures on mortality. Information derived from this study will be used to optimize routine clinical management and improve clinical care strategies. These will then help in the direction of health resources and improve cost effectiveness. It can also help validate outcome definitions, allow comparability of results and form a more accurate basis for patient allocation in further clinical studies.
The authors have no potential conflict of interest