- (2014) Volume 15, Issue 6
Masato Yoshioka1*, Go Watanabe1, Hiroshi Uchinami1, Norihito Ise1, Yasuhiko Nakagawa1, Kazuhiro Kudoh1, Ryo Morita2, Hideaki Andoh1 and Yuzo Yamamoto1
1Department of Gastroenterological Surgery and 2Cardiovascular and Respiratory Medicine, Akita University Graduate School of Medicine, 1-1-1 Hondo, Akita 010-8543, Japan
Received August 18th, 2014 – Accepted October 25th, 2014
Context Erlotinib is a selective epidermal growth factor receptor tyrosine kinase inhibitor used as a target therapy against non-small lung cancer and advanced pancreatic cancer. A regimen of erlotinib plus gemcitabine has been proven to prolong overall survival in the patient with advanced pancreatic cancer. In addition to common adverse effects, such as diarrhea, mucositis and skin rash (acne form eruptions), acute interstitial lung disease (ILD) has been reported as an infrequent but potentially fatal complication. We here report a case of a Japanese patient with erlotinib-induced ILD in whom high-dose corticosteroid therapy was successful. Case report A fifty five-year-old male with cancer of the head of the pancreas with multiple liver metastases started treatment with gemcitabine plus erlotinib. On the 13th day of erlotinib treatment, he had high fever. Chest computed tomography (CT) scan showed a diffuse ground-glass like infiltration of bothlungs. He was diagnosed with ILD, and high-dose corticosteroid therapy was started. Two weeks after the introduction of steroid therapy, the reticular shadow faded away on CT. He was successfully treated with corticosteroid for erlotinib-induced acute ILD although he died 6 months after the initiation of chemotherapy owing to disease progression. Conclusion we showed a case of a successfully treated Japanese patient of erlotinib-induced ILD. Because erlotinib-induced ILD would frequently occur in Japanese patients, closer attention to ILD should be paid for Japanese patients than in Western populations. If erlotinib-induced ILD occurs, a high-dose corticosteroid therapy would be a useful option of treatment.
Erlotinib; Gemcitabine; Lung Diseases, Interstitial; Pancreatic Neoplasms
Gemcitabine became a standard treatment for advanced pancreatic cancer since its superiority over fluorouracil was shown [1]. Since then, trials of many agents in combination with gemcitabine have failed to show survival improvement compared with gemcitabine alone [2, 3]. In contrast to these unsuccessful strategies, a regimen of erlotinib (Tarceva®, F. Hoffmann-La Roche AG, Basel, Switzerland) plus gemcitabine has been proven to prolong overall survival [4].
Erlotinib is a selective epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) used as a target therapy against non-small lung cancer (NSCLC) and advanced pancreatic cancer [5, 6]. Erlotinib is well tolerated and carries fewer adverse effects than most cytotoxic drugs. Among these EGFR-TKI agents, including gefitinib and erlotinib, common dose-limiting toxicities are diarrhea, mucositis and skin rash (acne form eruptions).
In addition to these common adverse effects, acute interstitial lung disease (ILD) has been reported as an infrequent but potentially fatal complication [7]. Gefitinib- associated ILD reportedly occurs in approximately 1% of patients worldwide [8], but only a few case reports have been published with erlotinib [7, 9].
We here report a case of a Japanese patient with erlotinib- induced ILD in whom high-dose corticosteroid therapy was successful.
A fifty five-year-old male had been admitted to our hospital for pancreatic cancer. He was not a smoker and had no history of pulmonary disease. An abdominal computed tomography (CT) scan revealed a tumor measuring 30 mm in diameter at the head of the pancreas (Figure 1a) with multiple liver metastases (Figure 1b, 1c). He was treated with gemcitabine in combination with erlotinib. Gemcitabine was administered with a protocol of 1,000 mg/m2 weekly for 3 weeks followed by 1 week rest. Erlotinib was administered with a protocol of 100 mg/body every day. One week after starting erlotinib, he developed a skin rash-mainly affecting the face and neck-which was controlled by oral minocycline without worsening. On the 13th day of erlotinib treatment, he presented with a fever elevated up to 40 degrees. No clinical focus of infection was found. Vital signs were stable, white blood cell (WBC) count was within normal limits 6,400 μL-1; reference range: 0-9,000 μL-1, and C-reactive protein (CRP) was slightly elevated (1.98 mg/dl; reference range <0.1 mg/dl). On the next day, the high fever continued and WBC count elevated to 11,800 μL-1. CRP level was also elevated to 16.09 mg/ dl. The percentage of blood eosinophil was within normal limits (1%; reference range 2–6%). Serum KL-6 level was within normal limits (448 U/ml; reference range <499 U/ ml). Chest X-ray revealed reticular shadow in the bilateral lung fields. Chest CT scan showed a diffuse ground-glass like infiltration of both lungs without any sign of pulmonary edema or pleural effusions (Figure 2a, 2b). He was diagnosed with ILD, and a high-dose corticosteroid with an antibiotic agent therapy was introduced. Prednisolone was applied with an initial dose of 1,000 mg/day for 3 days and an antibiotic agent was also applied for 3 days. After that, the reticular shadow was reduced gradually on CT. Following a high-dose corticosteroid pulse therapy, prednisolone was applied for internal use with a dose of 60mg/day. Two weeks after the introduction of steroid therapy, the ILD faded away on CT (Figure 2c, 2d). The dose of corticosteroid was gradually decreased by half every 2 weeks. Corticosteroid therapy was continued for 10 weeks. For detection of allergic response against erlotinib and gemcitabine, drug-induced lymphocyte stimulation test (DLST) was performed 4 weeks after occurrence of ILD. The stimulation indices were 84% of the control in gemcitabine and 88% in erlotinib, and therefore judged as negative.
Thereafter, he received chemotherapy with S-1, an oral fluoropyrimidine, administered at 80 mg/m2/day for 2 weeks followed by a 1 week rest. Six weeks after starting S-1 therapy, the tumor of the pancreas head was enlarged to 37 mm in diameter, suggesting that S-1 was ineffective. Although we could not rule out a possibility of gemcitabine as the cause of ILD, we again tried gemcitabine complying with the patient’s strong desire. Before the administration of gemcitabine, we repeatedly explained the risk of recurrence of ILD and took an informed consent. After re- starting gemcitabine, ILD did not occur again. Gemcitabine was continued for 6 weeks, but the tumor further enlarged to 51mm in diameter. Six months after the initiation of first chemotherapy, he died of disease progression.
Human epidermal growth factor receptor type 1 (HER1/ EGFR) is over-expressed in many pancreatic tumors [10, 11] and associated with poor prognosis and disease progression [12, 13]. Blocking HER1/EGFR TK signaling decreases the growth and metastasis of human pancreatic tumor xenografts [14] and enhances anticancer effects of gemcitabine [15]. In gastrointestinal oncology, erlotinib is currently applied in combination with gemcitabine as a first-line therapy for advanced-stage pancreatic cancers [6]. In advanced pancreatic cancers, such as recurrence post surgery or inoperable situations, gemcitabine and S-1 are commonly used for chemotherapy against metastasis and chemoradiotherapy against locally advanced tumors; however, newly developing molecular-targeting drugs like erlotinib are strongly anticipated for their effect against pancreatic cancers. Although the precise mechanism of toxicity remains to be elucidated, impaired metabolism of erlotinib is assumed to aggravate erlotinib toxicity [16]. In humans, erlotinib is metabolized by cytochrome P450 enzymes, predominantly by CYP3A4 and to a lesser part by CYP1A2 and CYP1A1 [17]. The reactive intermediates formed during erlotinib metabolism are cellular oxidases, which may produce oxidative stress by modifying cellular proteins. The oxidative stress potentially contributes to the clinically observed toxicities [16, 17].
The phase III trial by the National Cancer Institute of Canada Clinical Trials Group, which led to approval of erlotinib in combination with gemcitabine for advanced pancreatic cancers, reported an incidence of ILD-like toxicities as 2.1% in the patients treated with erlotinib plus gemcitabine versus 0.4% in the patients treated with placebo plus gemcitabine [4]. There is no randomized controlled trial to guide the management of EGFR-TKI agent-induced ILD. Previous policy of management for EGFR-TKI agent-induced ILD was to discontinue this medication and to start oxygen therapy. However, several researchers indicated that a high-dose steroid treatment was effective in ILD caused by gefitinib [18].
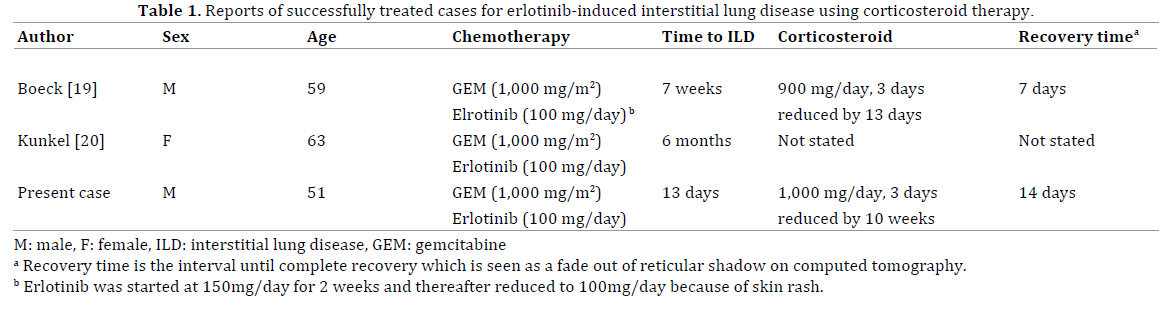
With regard to the successful treatment by corticosteroid therapy for erlotinib-induced acute ILD in pancreatic cancer patients, there are only 2 case reports in the English literature thus far (Table 1). Boeck et al. reported the first case of successful treatment of ILD in a patient with metastatic pancreatic cancer [19]. This patient had taken gemcitabine plus erlotinib (150 mg daily) treatment within the phase III trial. In this report, because a severe skin rash had occurred after 2 weeks of treatment, erlotinib was temporally discontinued for 1 week. Thereafter, treatment had re-started with a reduced dose of 100 mg/ daily for erlotinib. Seven weeks after the initiation of chemotherapy, acute ILD had occurred. He was treated with a high-dose steroid (prednisolone) therapy with an initial dose of 900 mg/day for 3 days. A CT scan 7 days after introducing steroid therapy showed a complete recovery from ILD. Kundel et al. reported the second case [20]. This patient was treated with 1,000 mg/m2 gemcitabine weekly and 100 mg of erlotinib daily for pancreatic cancer and hepatic metastases. After 6 months of therapy, acute ILD had occurred. Following the corticosteroid therapy, a complete resolution of acute ILD was confirmed on CT scan. Unfortunately, the dose of corticosteroid was not stated in this report.
Concerning the susceptibility to EGFR-TKI drug-associated ILD with respect to race, ILD occurred only in 2.4% of pancreatic cancer patients who were treated with gemcitabine plus erlotinib according to Moore et al. [4]. On the other hand, in the similar study in Japanese patients by Okusaka et al. [21], ILD-like events occurred in 9 patients from 107 patients (8.5%). This higher incidence in Japanese patients is authenticated by the post-marketing surveillance of erlotinib in Japan, in which the incidence of ILD-like events was computed as 6.1% in 313 patients [22]. These two reports implied that the incidence of erlotinib-associated ILD is much higher in the Japanese population than Western populations. Similar difference of susceptibility is observed in gefitinib. According to the U.S. Food and Drug Administration (FDA) drug approval summary, the incidence of gefitinib-associated ILD was 2% in Japanese patients and about 0.3% in the United States patients [8]. Moreover, in the study of Takano et al., the incidence of gefitinib-induced ILD in Japanese patients was as high as 5.4% [23]. Concerning the difference of incidence in Japanese patients between the FDA summary and Takano’s study, Takano gave his opinion that many ILD cases might not have been reported because a follow-up survey of all cases had not been conducted. Nevertheless, gefitinib-induced ILD also occurs more frequently in the Japanese population than the Western population. Taken together, Japanese patients might have a higher risk for the incidence of ILD toward EGFR-TKI drugs.
The mechanism of EGFR-TKI-induced ILD is still not elucidated. EGFR has important roles in the maintenance and repair of epithelial tissues by regulating cell migration, differentiation, proliferation and survival. EGFR is expressed in type II pneumocytes, which are responsible for recovery of normal alveolar architecture following alveolar injury. Higenbottam et al. postulated that inhibition of EGFR would partly reduce the pneumocyte capacity responding to lung injury [24]. Because the ILD in our patient occurred rapidly on the 13th day of erlotinib treatment, drug hypersensitivity was a possible mechanism. Although we failed to examine Ig-E levels, acute hypersensitivity reaction was very improbable. This was the first time the patient had ever received erlotinib and his blood eosinophil count did not alter at all during the course, remaining in the normal range. In relation to delayed hypersensitivity as well, the DLST result was negative. Therefore we deem that the allergic mechanism was unlikely in our patient. At present, the exact reason why ILD was induced by erlotinib in this patient is not uncovered but causal relationship between the ILD and erlotinib is strongly inferred from his clinical course.
In conclusion, we showed a case of a successfully treated Japanese patient of erlotinib-induced ILD. Because erlotinib-induced ILD would frequently occur in Japanese patients, closer attention to ILD should be paid for Japanese patients than in Western populations. If erlotinib-induced ILD occurs, a high-dose corticosteroid therapy would be a useful option of treatment.
Authors declare to have no conflict of interest.