- (2005) Volume 6, Issue 2
Christophe E Pierreux, Aurélie V Poll, Patrick Jacquemin, Frédéric P Lemaigre, Guy G Rousseau
Hormone and Metabolic Research Unit, Institute of Cellular Pathology and Université Catholique de Louvain, Brussels, Belgium
Received December 17th, 2004 - Accepted January 27th, 2005
Context Understanding gene function in the developing pancreas is a major issue for pancreatic cell therapy. The in vivo analysis of gene function has essentially been performed by modulating gene expression in transgenesis. A faster and easier method is electroporation of mouse embryos. This technique, coupled with whole embryo culture, enables one to deliver genes and analyze their effects in a spatially and temporally regulated manner. Objective We wanted to adapt the electroporation technique for gene transfer of whole e8.5 mouse embryos into the endoderm to allow expression of transgenes in the pancreas or liver. Results Using two platinum plate electrodes, low voltage and a precise positioning of the embryo in the electroporation cuvette we could target and express DNA constructs in the prepancreatic or prehepatic territories, identified with cell markers. We also demonstrated that this technique is a valuable tool in the study of transcriptional regulation in the developing endoderm. Conclusions Targeted electroporation of whole embryos is a useful method of characterizing the gene network which controls pancreatic development.
Electroporation; Embryo Culture Techniques; Liver; Mice; Pancreas; Transcription, Genetic
CMV: cytomegalovirus; GFP: green fluorescent protein; HBSS: Hank's balanced salt solution; HNF: hepatocyte nuclear factor; LUC1: firefly luciferase; LUC2: renilla luciferase; Pdx-1: pancreasduodenum- homeodomain
During embryonic development, pancreatic precursors appear in a specific region of the endoderm, the prepancreatic endoderm. Cells in this region proliferate, bud off from the endoderm and differentiate to generate the mature pancreas. Analysis and understanding of the gene function in the developing pancreas is a prerequisite for pancreatic cell therapy. A standard strategy for analyzing gene function in mammalian developmental biology is transgenesis in the mouse. However, this technique is time-consuming and the transgene may be lethal, resulting in premature death before reaching the desired stages. Moreover, the spatio-temporal control of transgene expression is not always possible, due to the lack of specific promoter elements. A faster and easier way to analyze gene function is electroporation [1, 2]. The electric pulse creates membrane pores in the cell through which the negatively charged nucleic acids (DNA, mRNA, siRNA) penetrate while moving towards the anode. This technique allows the introduction of constructs which: i) overexpress genes (gain of function); ii) silence gene expression or code for dominant negative factors (loss of function); or iii) display the transcriptional activity of regulatory regions (transcriptional assay). Developmental biologists have widely used in ovo chick electroporation to achieve ectopic gene expression mainly because avian embryos can be reached easily and subsequently cultured [3].
Electroporation of mouse embryos is more complicated, as the conceptus must be removed from the uterus beforehand. After in vitro electroporation, the whole embryo can be incubated under specific culture conditions [4] which allow it to continue its growth and morphogenesis. The electroporated construct can be expressed in a temporally and spatially regulated manner, as embryos can be dissected out at different stages and cultured in vitro from the pregastrula to the early organogenesis stages [5]. Electroporation can also target a specific site of the embryo if one correctly positions the tissue in the path of movement of the DNA towards the anode [6, 7]. When we started this study, successful electroporation of e8.5 and e9.5 mouse embryos had been reported, but only the ectoderm had been targeted [8, 9]. In a recent paper, Tam et al. [10] reported that they could electroporate the endodermal cells of the mouse gastrula (e7.5) with a green fluorescent protein (GFP)-expression vector to track the fate of the transfected cells in the early somite (e8.0)-stage embryo. However, the precise targeting of discrete regions of mammalian endoderm in order to allow the expression of genes at later stages in endoderm-derived organs, such as the pancreas or liver, was not described. We have adapted the technique of electroporation to deliver genes in the midgut region of the endoderm of e8.5 mouse embryos and to follow the expression of the transgenes in endoderm-derived organs. This region is of particular interest because it gives rise to the pancreas and he liver. The development of these organs is being studied extensively in order to unravel the gene regulatory networks which control the differentiation of multiple cell lineages from common pluripotent precursors. Here, we show that electroporated constructs can be targeted to the prepancreatic and prehepatic endoderm, following which the embryo can be cultured to study transgene expression in the developing pancreas and liver. We further show that this technique permits the study of transcriptional regulation in vivo.
Mouse Embryo Collection, Culture and Observation
Twenty-four mice were used in this study. Mouse embryos were obtained from pregnant CD1 mice at 8.5 days postcoitum (e8.5) and placed in Hank's balanced salt solution (HBSS). The conceptus was taken out of the uterus and the Reichert's membrane was removed. After electroporation, embryos were cultured for 24 h at 37°C in a roller culture system (31 rpm). The culture medium was composed of HBSS or DMEM (Invitrogen, Merelbeke, Belgium), supplemented with 50% inactivated rat serum and equilibrated with 5% O2/5% CO2/90% N2. Visualization of GFP activity in live embryos was done under a fluorescence microscope (Axiovert 200, Zeiss, Jena, Germany) and pictures were taken using a digital camera (Coolpix 995, Nikon, CYPAC, Brussels, Belgium).
Plasmids
In the pcDNA3-EGFP vector (Invitrogen, Merelbeke, Belgium), the cytomegalovirus (CMV) promoter drives expression of the GFP reporter protein. The hepatocyte nuclear factor-6 (HNF-6) expression vector and HNF- 6-responsive firefly luciferase (LUC1) reporter vector have been described [11]. The internal reporter control (renilla luciferase; LUC2) was pRL-CMV (Promega, Madison, WI, USA). Plasmid DNA, prepared using the QIAGEN® plasmid extraction kit, was resuspended in HBSS to obtain concentrations of at least 1.5 μg/μL.
Electroporation
Electroporation was preceded by incubation of the embryos with the plasmid solution so that the DNA can be adsorbed on the surface of the endoderm. Dissected embryos were then transferred to a 30 μL drop of plasmid solution (concentration from 0.5 to 1.5 μg/μL) in a bacterial culture dish and incubated for 10 min at room temperature. Embryos were transferred with the DNA solution in the electroporation cuvette between the two platinum plate electrodes (3x8 mm) (CUY520P5, Protech International, San Antonio, TX, USA) placed 5 mm apart. To target the prepancreatic endoderm, embryos (6- to 8-somite) were oriented in the cuvette so that the ectoplacental cone was tilted at an angle of approximately 45 degrees to the anode. To target the prehepatic endoderm, younger (4- to 6-somite) embryos were used and oriented in a similar way. Electroporation was performed using a square-wave pulse generator (Electro Square Porator ECM 830, BTX; San Diego, CA, USA). Embryos were viable and developed correctly when three 50 ms pulses of 9 V were applied at 1 s intervals.
Histology and Immunofluorescence
Cultured embryos were fixed in 4% paraformaldehyde for 1 h on ice. The embryos were embedded and immunofluorescence was detected as described [12]. Primary antibodies and dilutions were as follows: monoclonal mouse anti-E-cadherin at 1:50 (BD Transduction Laboratories, Erembodegem, Belgium), rabbit anti-GFP at 1:100 (Molecular Probes, Eugene, OR, USA), rabbit anti-Pdx-1 at 1:1,000 (a kind gift from CV Wright), and rabbit anti- Prox1 at 1:4,000 (Covance, Princeton, NJ, USA). Sections were analyzed with a fluorescence microscope (Axiovert 200, Zeiss, Jena, Germany) and pictures were taken using a digital camera (Coolpix 995, Nikon, CYPAC; Brussels, Belgium).
Luciferase Activity
For luciferase assay, the endoderm was dissected out of the embryo after 24 h in culture and homogenized in passive lysis buffer (Promega; Madison, WI, USA) according to the instructions of the manufacturer. Luciferase activities were measured using the Dual Luciferase kit (Promega; Madison, WI, USA) and a TD- 20/20 luminometer (Promega; Madison, WI, USA).
ETHICS
Mice were treated according to the principles of laboratory animal care of the Université Catholique de Louvain Animal Welfare Committee.
STATISTICS
Relative luciferase activity (LUC1/LUC2) is reported as mean±SEM. The Student's t-test was applied. A two-tailed P value of 0.05 was chosen to define statistical significance.
RESULTS
Embryo Survival and Development after In Vivo Electroporation Which Targets the Endoderm
Electroporation of the ectoderm requires injection of the constructs into the amniotic cavity and then positioning of the embryo so that the ectoderm faces the cathode. In contrast, the endoderm is exposed to the external environment once the embryo has been removed from the uterus and cleared from the decidua and Reichert's membrane. Thus, the endoderm is directly accessible and it can be targeted by bathing the embryo in a DNA solution with the endoderm facing the cathode, towards which the negatively charged DNA will move (Figures 1A and 1B). The only drawback of this procedure is that it requires higher quantities of DNA. In our experiments, the embryo was bathed for 10 min in a solution of HBSS containing 0.5- 1.5 μg/μL of plasmid DNA, transferred between the two electrodes, and electroporated. To target the prospective midgut (ellipse with blue stippling in Figure 1A), the embryo was slightly tilted such that this region was facing the cathode (Figure 1B). To monitor the efficiency of electroporation and endoderm targeting, the plasmid electroporated contained reporter gene coding for GFP driven by the CMV promoter. This promoter is known to promote vigorous and ubiquitous transcription of reporter genes in electroporated mouse embryos [7]. The embryo was then cultured for 24 h as described in the "Methods" section. When one works on whole embryos, electroporation efficiency must be balanced against embryo viability since electric shocks may result in cell death. Similarly, embryo development should not be affected by the electroporation conditions. In this respect, long square pulses of low voltage appear to be preferable [1, 9]. Based on conditions described by Davidson et al. [7], we used a square-wave pulse generator and two platinum plate electrodes, allowing administration at a low voltage. Embryo viability and development in culture were clearly dependent on the voltage (Table 1) and the number of pulses (data not shown) applied. At 12 V or more, embryo development was disturbed, with defects such as heart hypertrophy or dorsal kink opposite to the targeted region. In our studies, good viability, correct development and efficient electroporation (see below) were obtained with three 50 ms pulses of 9 V at 1 s intervals (Figure 1C). After gastrulation, the sheet of cells which forms the endoderm gives rise to the gut tube. This involves turning of the embryo, closure of the midgut region, and formation of the primitive gut tube by e9.0 [13]. As shown in Figure 1D, these morphogenetic events did occur properly during the culture. In our hands, 6- to 8- somite embryos cultured for 24 h reached the 18- to 22-somite stages. We concluded that our electroporation conditions did not alter embryo survival but affected the development of some embryos. These abnormal embryos were discarded.
Figure 1. Electroporation and culture of e8.5 mouse embryos. A. The embryo (drawn here without the visceral yolk sac and amnion) in the electroporation cuvette between the cathode and the anode to target the gut endoderm. The movement of the negatively charged DNA molecules (green) is indicated by the arrow. The region targeted by the DNA is shown in stippled blue. B. Picture and orientation of the embryo (8-somite stage) in the electroporation cuvette prior to electric pulses. C. The square wave pulses used for electroporation of e8.5 embryos. D. Development of an embryo electroporated at the 8-somite stage and cultured for 24 h. Note the progress in development as compared to the embryo shown in panel B.

Expression of the Electroporated Vector in the Prepancreatic or Prehepatic Epithelium
As the embryo develops as it does in vivo within the 24 h culture, the cells of the endodermal layer, which is still external in the e8.5 embryo, become internalized during the culture to form the gut tube. To follow the fate of the cells electroporated under the conditions selected, the embryos were electroporated with the GFP-expression vector. After the 24 h culture, they were dissected out of the visceral yolk sac and amnion, and GFP activity was visualized on live embryos. As shown in Figure 2A, GFP activity was clearly visible in the midgut region. To confirm that these GFP-positive cells were localized in the newly formed gut tube, a GFP-electroporated embryo was fixed and immunofluorescence was detected on the paraffin-embedded embryo. Sections were costained with antibodies against GFP and against E-cadherin, which is a component of the adherens junction complex found between epithelial cells. E-cadherin antibody staining permits localizinf the endoderm-derived columnar epithelium and then distinguishing it from the surrounding nonepithelial tissues. As shown in Figure 2B, GFP staining colocalized with the E-cadherin staining, indicating that the GFP-positive cells were localized in the epithelium. Hoechst staining of the whole embryo (Figure 2B) showed that the electroporated territory corresponds to a region of the primitive gut from which the pancreas and liver are expected to bud off. We conclude that our electroporation conditions and embryo orientation permit the delivery of a GFP-expressing vector in a discrete region of the developing endoderm which presumably corresponds to prepancreatic and/or prehepatic tissue. In the e9.0 primitive gut, several transcription factors are expressed within specific regions corresponding to organ-specific domains. Pancreas-duodenum-homeodomain protein (Pdx-1), a marker of the pancreatic territory, is required for pancreatic bud expansion [14, 15]. Another territory-restricted transcription factor is Prox1, whose expression is detected in the hepatic endoderm (e8.5) [16]. To verify that prepancreatic and/or prehepatic tissues were targeted in our electroporation experiments, we performed immunofluorescence studies using antibodies directed against GFP, Pdx-1, or Prox1. Figures 3A-F show adjacent sections stained with anti- GFP/E-cadherin (Figures 3A and 3D), anti- Pdx-1 (Figures 3B and 3E) or anti-Prox1 (Figures 3C and 3F) antibodies. These showed that the dorsal pancreatic region, detected with the Pdx-1 antibody, exhibited strong and extended GFP staining. In that dorsal region, almost all the Pdx-1 positive cells were electroporated. The prehepatic region, as revealed by Prox1 reactivity, and the lateral region of the endoderm showed scattered cells reacting with the GFP antibody. On the other hand, preferential electroporation of the prehepatic territory could be achieved by using younger (4- to 6- somite, rather than 8-somite) embryos (data not shown). These experiments demonstrate that, by electroporating a whole embryo, one can deliver an exogenous construct in the prepancreatic or prehepatic endoderm.
Figure 2. Gene targeting of a restricted region of the mouse endoderm by whole embryo electroporation. A. Overlay of bright field and fluorescence images of an embryo electroporated at the 8-somite stage with a GFP expression vector and cultured for 24 h, showing GFP activity (green) only in the midgut region. B. Immunolocalization of GFP (green) in the E-cadherin (red)-positive epithelium of the primitive gut. The GFP positive region is boxed in the lower panel, which shows Hoechst staining of a section of the whole embryo.
Figure 3. Gene targeting of the prepancreatic and prehepatic territories of the mouse endoderm. A-C and D-F. Two
series of adjacent sections of the same embryo electroporated at the 8 somite stage and cultured for 24 h. Sections
stained with antibodies against GFP and against the epithelial marker E-cadherin (A, D), against the pancreatic marker
Pdx-1 (B, E), or against the hepatic marker Prox1 (C, F), showing that GFP was delivered to the prepancreatic region.
dpb: dorsal pancreatic bud
hb: hepatic bud
Transcriptional Assay in the Endoderm of Electroporated Embryos
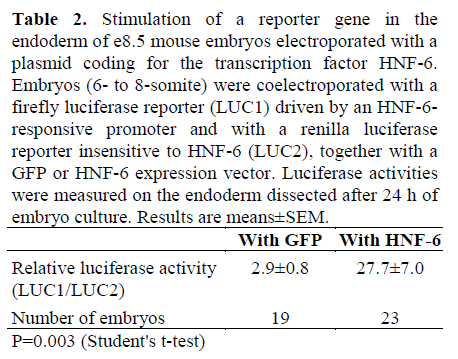
Using electroporation, we could modulate the endogenous gene expression program in the endoderm by overexpression of a transcription factor [17]. We have now tested whether we could adapt this technique to study transcriptional regulation in the endoderm. The experiments were designed to coexpress a transcription factor and a reporter gene driven by a promoter which can be stimulated by this transcription factor. The factor chosen was HNF-6, whose endogenous expression is first detected in mouse endoderm around e8.0 [18]. Later on, HNF-6 is expressed mainly in the pancreas and liver. The reporter was a luciferase construct (LUC1) whose expression depends on the binding of HNF-6 to its promoter [11]. A second reporter plasmid (LUC2), whose expression is independent of HNF-6, was also electroporated as a control of transfection efficiency. Embryos in which these two reporters were coelectroporated with an expression vector coding for GFP instead of HNF-6 were studied in parallel. After 24 h in culture, the endoderm was dissected out and luciferase activities were measured. As shown in Table 2, relative luciferase activity was about 10 times higher in the embryos coelectroporated with the HNF-6 expression vector than in those coelectroporated with the GFP expression vector.
CONCLUSIONS
The technique described here permits the delivery of DNA constructs in the prepancreatic and prehepatic endoderm of early somite-stage mouse embryos. The tissue targeted depends on the developmental stage and orientation of the embryo between the electrodes. Construct coding for transcription factors, intracellular or extracellular signaling molecules, cell surface receptors or their dominant negative counterparts, could thus be electroporated for gain or loss of function studies in the endoderm. This technique should help in understanding the genetic program which governs pancreatic development. It could also be useful for the identification or mapping of regulatory gene regions by fusing them to a reporter gene (GFP, beta-galactosidase or luciferase), as a first step before the more demanding transgenic methods. Moreover, the electroporated construct has no size restriction and very large gene regions such as bacterial artificial chromosomes could be analyzed. Finally, electroporation of the endoderm coupled with whole embryo culture enables one to study transcriptional control in the developing endoderm rather than in cultured cells [17].
The authors are grateful for the advice of W. Lin, S.L. Ang, and P. Tam. They also thank C.V. Wright for a generous gift of anti-Pdx-1 antibody, J. van Eyll and F. Clotman for discussion, and S. Cordi and J.F. Cornut for technical help. This study was supported by grants from the Belgian State Program on Interuniversity Poles of Attraction, from the D.G. Higher Education and Scientific Research of the French Community of Belgium, and from the Fund for Scientific Medical Research. C.E.P. was a Senior Research Assistant of the 'Fonds National de la Recherche Scientifique' (FNRS); A.V.P. holds a fellowship from the 'Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture' (FRIA); P.J. is Research Associate of the FNRS.