- (2007) Volume 8, Issue 4
Muhammad Wasif Saif1, Armin Shahrokni2, Daniel Cornfeld1
1Yale University School of Medicine. New Haven, CT, USA
2Griffin Hospital. Derby, CT, USA
Received March 29th, 2007 - Accepted May 21st, 2007
Gemcitabine is the only cytotoxic agent approved by FDA for the treatment of pancreatic carcinoma. Gemcitabine has a relatively safe profile. Major side effects include bone marrow suppression and flu-like syndrome. Transient abnormalities of liver transaminase enzymes are seen in two third of patients: elevations of alkaline phosphatase and bilirubin are less common, but severe hepatic toxicity is uncommon. Four case reports regarding severe hepatic toxicity of gemcitabine leading to rapid deterioration in patients’ health status and death have been reported. We report the fifth case in which liver functions were within normal limits but liver toxicity was preceded by radiological findings on the MRI. We describe a 61-year-old male with stage T4N1M0 who initially received gemcitabineoxaliplatin (GemOx) regimen was switched to gemcitabine-capecitabine (every two weeks schedule) after four months of therapy due to lack of response. Restaging CT scan after eight-weeks showed new multiple foci of low attenuation resembling simple cysts. MRI of the abdomen was performed which revealed early and active fibrosis. Hepatitis panel were negative. Subsequently the patient developed nausea, vomiting, abdominal pain and weight loss and was referred for palliative radiotherapy. Gemcitabine was discontinued and follow-up CT scan two months later showed stable lesions in the liver. In conclusions, four cases of gemcitabineinduced liver toxicity has been reported in the literature. Such toxicity is manifested by elevated liver transaminases and more common in the presence of liver metastasis. However, our case showed that gemcitabineinduced liver toxicity can be detected by MRI, before liver enzymes start to rise and discontinuation of gemcitabine can prevent further liver toxicity and fibrosis. Report of such cases is encouraged as it will bring awareness among clinicians caring for such patients receiving gemcitabine.
gemcitabine; Liver Cirrhosis; Magnetic Resonance Imaging; Pancreatic Neoplasms; Transaminases
Gemcitabine is the only cytotoxic agent approved by FDA for the treatment of pancreatic carcinoma. Gemcitabine has a relatively safe profile. Major side effects include bone marrow suppression and flu-like syndrome. Transient abnormalities of liver transaminase enzymes are seen in two third of patients: elevations of alkaline phosphatase and bilirubin are less common, but severe hepatic toxicity is uncommon. Four case reports regarding severe hepatic toxicity of gemcitabine leading to rapid deterioration in patients’ health status and death have been reported. We report the fifth case in which liver functions were within normal limits but liver toxicity was preceded by radiological findings on the MRI.
We describe a 61-year-old male with stage T4N1M0 who initially received gemcitabineoxaliplatin (GemOx) regimen was switched to gemcitabine-capecitabine (every two weeks schedule) after four months of therapy due to lack of response. Restaging CT scan after eight-weeks showed new multiple foci of low attenuation resembling simple cysts. MRI of the abdomen was performed which revealed early and active fibrosis. Hepatitis panel were negative. Subsequently the patient developed nausea, vomiting, abdominal pain and weight loss and was referred for palliative radiotherapy. Gemcitabine was discontinued and follow-up CT scan two months later showed stable lesions in the liver.
In conclusions, four cases of gemcitabineinduced liver toxicity has been reported in the literature. Such toxicity is manifested by elevated liver transaminases and more common in the presence of liver metastasis. However, our case showed that gemcitabineinduced liver toxicity can be detected by MRI, before liver enzymes start to rise and discontinuation of gemcitabine can prevent further liver toxicity and fibrosis. Report of such cases is encouraged as it will bring awareness among clinicians caring for such patients receiving gemcitabine.
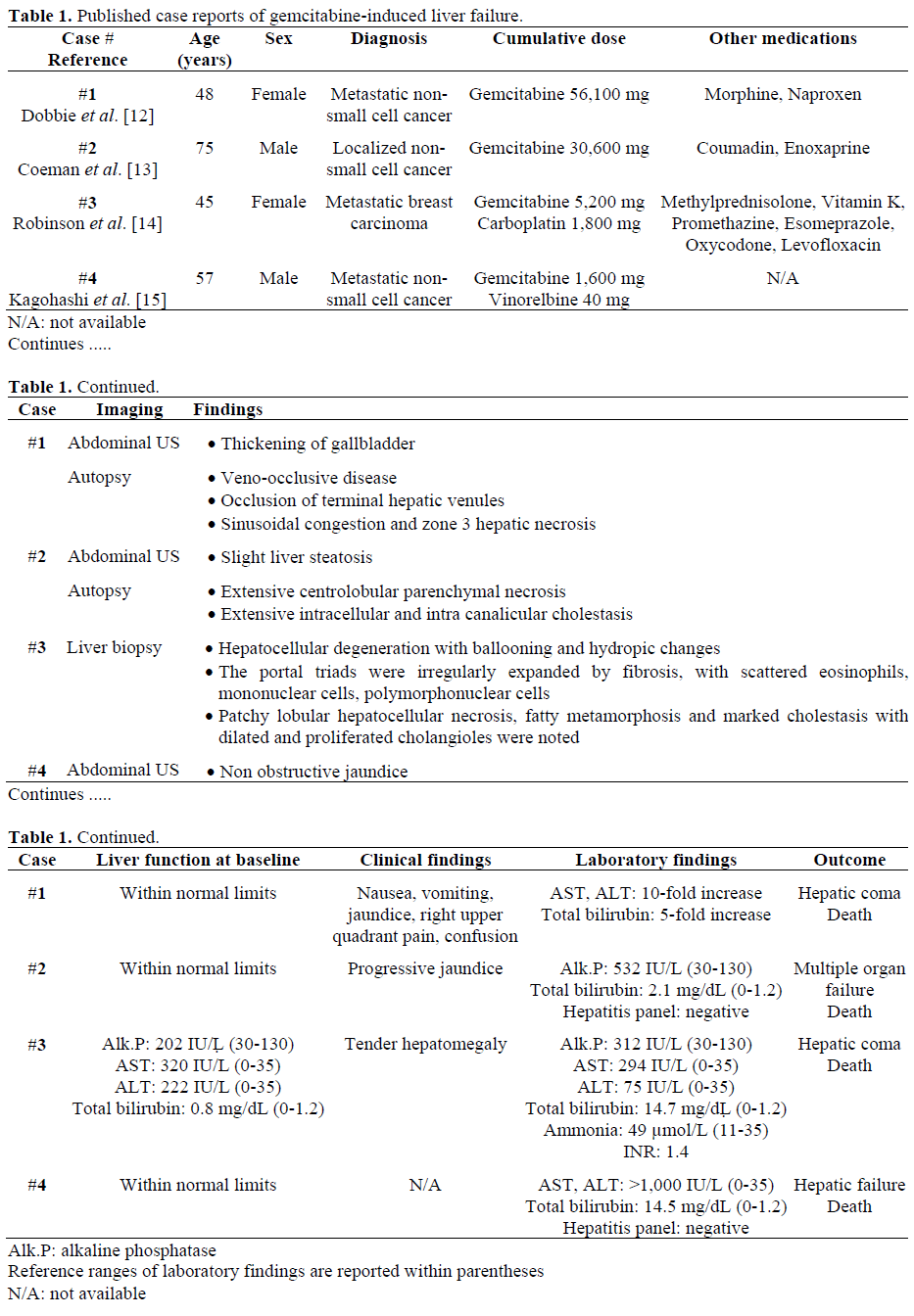
Gemcitabine (difluorodeoxycytidine; dFdC) is the only cytotoxic agent approved by FDA for the treatment of advanced pancreatic carcinoma. It is also useful in non small cell lung carcinoma, breast, urothelial and ovarian cancer [1]. Major toxicities of gemcitabine include marrow suppression, and flu-like symptoms. Less common toxicities include (but not limited to): radiation recall [2], erysipeloid skin toxicity [3], acute myocardial infarction [4], atrial fibrillation [5], interstitial pneumonitis [6], respiratory failure [7], hemolytic uremic syndrome [8], severe neurotoxicity [9], vasculitis [10] and reactivation of hepatitis B [11]. Four cases of gemcitabine-induced liver failure have been reported. [12, 13, 14, 15]. Patients’ characteristics, cumulative dose of gemcitabine, liver function status at baseline, laboratory data, imaging findings, biopsy findings (if performed) and outcome are described in Table 1. None of these patients have been diagnosed with pancreatic cancer. We, here, present the first patient with pancreatic adenocarcinoma who developed liver fibrosis during treatment with gemcitabine and capecitabine.
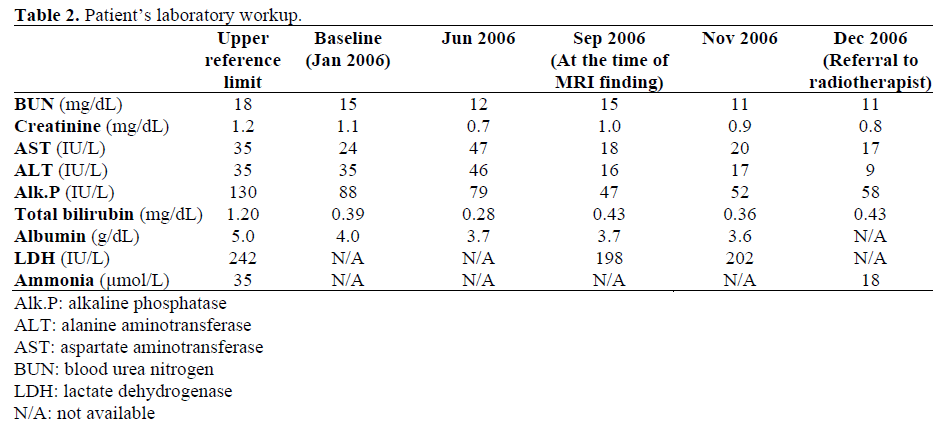
weeks with capecitabine given as 2,000 mg twice daily for seven out of fourteen days. Restaging CT scan after eight weeks showed new multiple foci of low attenuation resembling simple cysts. To further evaluate these findings on the abdominal CT scan, an MRI of the abdomen with and without gadolinium was performed, which showed multiple simple cysts within liver parenchyma, largest being within lateral segment of the left lobe, and might represent peliosis. On arterial phase post contrast sequence, there was enhancement seen along the periphery within the liver most prominently within the posterior segment of right lobe which persisted on delayed sequences. These findings were most suggestive of early and active fibrosis (Figures 1, 2, 3, and 4). Liver function tests were within the normal limits at the baseline and remained normal throughout the whole course of chemotherapy cycles (Table 2). They were normal also at the time of MRI findings. Hepatitis panel was negative. History and examination did not suggest any rheumatologic or infectious etiology. A biopsy was suggested but was not denied. Clinically, he also started deteriorating with worsening abdominal pain, nausea, vomiting and weight loss. Therefore, it was decided to stop gemcitabine-based chemotherapy. Due to persistent pain, the patient was subsequently referred for palliative radiotherapy. Follow-up CT scan in two months showed stable lesions in the liver and no definitive matastatic lesions.
Figure 2. Axial fast response fast spin echo, fat saturated, T2 weighted image (Flip/TR/TE 90/2000/73, slice 8 mm, field of view (FOV) 40, matrix 384x192) through the liver. Linear areas of increased signal intensity are present along the liver periphery (black arrows). This is a finding indicative of early or mild fibrosis. There is also increased signal along the course of several intrahepatic bile ducts (white arrows). While this could represent fluid within the ducts, there is also increased signal within the walls of the ducts (white arrowhead) which is indicative of inflammation or fibrosis.
Figure 3. Axial 3D spoiled gradient echo, fat saturated, T1 weighted image (Flip/TR/TE 12/4.25/1.9, slice 4 mm, field of view (FOV) 40, matrix 320x192) through the liver in the arterial phase (approximately 20 seconds after contrast injection) at the same level as Figure 2 above. There are linear areas of enhancement around the liver periphery (black arrows) which correspond to the linear areas of increased T2 signal in Figure 2. This indicates active inflammation. There is also arterial enhancement of the intrahepatic bile ducts (white arrows). The early enhancement indicates active inflammation.
Figure 4. Axial 3D spoiled gradient echo, fat saturated, T1 weighted image (Flip/TR/TE 12/4.25/1.9, slice 4 mm, field of view (FOV) 40, matrix 320x192) through the liver in the equilibrium phase (approximately 120 seconds after contrast injection) at the same level as in Figures 2 and 3. There is persistent enhancement of the linear areas of active liver fibrosis (black arrows) and within the walls of the intrahepatic bile ducts (white arrows). The biliary enhancement has become more prominent with time. The delayed enhancement is typical of fibrosis. The early enhancement seen in Figure 3 indicates that the process causing fibrosis is active. Of note, the proximal left portal vein (black arrowhead) is seen in this more delayed image. It was not opacified on the arterial phase image (Figure 3).
Gemcitabine (2',2'-difluorodeoxycytidine, dFdC) is a pyrimidine analog of deoxycytidine. Liver toxicity associated with gemcitabine has been reported mostly as a transient rise in transaminases which rapidly reverses and usually patients remain asymptomatic [16, 17]. Previously, there have been four case reports reporting serious liver toxicity secondary to gemcitabine. As described in Table 1, all these four cases have been reported on patients with non-pancreatic carcinoma [12, 13, 14, 15]. Three patients had either localized or metastatic non small cell carcinoma of lung and the fourth patient had matastatic breast carcinoma [14]. Liver function tests were within normal limit at baseline in three cases and abnormal in the fourth case patient (elevated alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase [14]. In two cases, patients received more than total of 30,000 mg of gemcitabine [12, 13] and in the other two cases patients received less than 6,000 mg of gemcitabine [14, 15]. In all four cases, patients presented with significant liver toxicity as manifested by significant increase in alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase and total bilirubin. In two cases, hepatitis panel has been checked and those were negative [13, 15]. Three patients have undergone either biopsy or autopsy [12, 13, 14]. In a case reported by Dobbie et al., [12] autopsy showed occlusion of terminal hepatic venules, sinusoidal congestion and zone-three hepatocyte necrosis which was consistent with veno-occlusive disease. Authors attributed the veno-occlusive disease to gemcitabine and considered the chronic HCV as a potential risk factor. Autopsy in a case that has been reported by Coeman et al., [13] showed intracellular and intra canalicular cholestasis without evidence of obstruction. Interestingly, autopsy showed preservation of liver architecture and extensive centrilobular parenchymal necrosis was acute because there was no collapse or approximation of portal fields. Authors have emphasized on considering gemcitabine as one of the causes of cholestasis in patients who are receiving gemcitabine either as a single therapy or in combination with other medication and chemotherapy medications. Biopsy that has been performed on a case being reported by Robinson et al., [14] showed irregular expansion of portal triads by fibrosis and marked cholestasis with dilated and proliferated cholangioles Autopsy revealed patchy lobular hepatocellular necrosis. The biopsy was compatible with cholestatic liver disease and it was consistent with druginduced hepatocellular injury. Subsequently autopsy has been performed and showed capsular metastasis and intrahepatic metastasis involving 10-15% of hepatic parenchyma. On the other hand, our patient was receiving lower dose than the patient described in this paper due to expected poor tolerance in US patients, a fact well-known to oncologists now. Our patient is the first patient with pancreatic carcinoma who developed radiological findings of liver fibrosis following treatment with gemcitabine. The current patient had received a total of 14,350 mg of gemcitabine and 1,435 mg of oxaliplatin. Additionally, he received another 12,452 mg of gemcitabine with 12,000 mg of capecitabine. A biopsy was suggested but was not performed. The dose was picked from a randomized phase II study published in 2003 [18]. This schedule was selected due to patient’s request as he lived distant and biweekly regimen was convenient. Similar biweekly regimen of gemcitabine has been safely tested in other studies and tumor types. Our patient received gemcitabinecapecitabine after GemOx combination failed to show response. In a randomized phase II clinical trial by Scheithauer et al., [18] that investigated the efficacy of gemcitabinecapecitabine in treatment of metastatic pancreatic adenocarcinoma, investigators found no grade III or IV hepatic toxicity in the combination arm. So it is very unlikely that combination of gemcitabine and capecitabine is responsible for liver fibrosis. Moreover, literature review and our extensive personal experience suggest that capecitabine can be safely administered to patients with cancer and liver dysfunction [16, 19, 20]. Generally, the pharmacokinetics of capecitabine are not affected in patients with mild to moderate hepatic dysfunction, but there are no data available for patients with severe hyperbilirubinemia. This was also found to be true in the UK-based study whichactually concluded that no dose adjustment of capecitabine was necessary in patient with mild to moderate hepatic dysfunction secondary to liver metastases [21]. Therefore, capecitabine was not believed to be responsible. The main toxicity of oxaliplatin, a major drug in the treatment of metastatic colorectal carcinoma, is neurologic. Severe sinusoidal lesions of the liver have been recently described in patients receiving preoperative oxaliplatin-containing chemotherapy, but their clinical relevance is unknown [22]. Oxaliplatin was stopped more than two months and no such findings were seen on CT scan both during and after oxaliplatin therapy. The clinical significance of this case really lies in the fact that sometimes, radiological findings may come before any clinical or laboratory abnormalities. Above all, stopping gemcitabine can reverse such changes. Radiological changes with normal liver function tests have been reported with other chemotherapeutic agents, e.g. methotrexate [23]. Causes of liver fibrosis are vast. Chronic hepatitis B and C can cause liver fibrosis. Cheong et al., [11] have reported a case of reactivation of hepatitis B after administration of gemcitabine. Hepatitis panel was negative in our case. Veno-occlusive disease of liver can be ruled out based on the setting of normal liver function tests through the whole course. Hepatotoxic drugs are generally categorized to be dose-dependent or predictable vs. dose-independent or unpredictable (idiosyncratic). Idiosyncratic reactions may be immunoallergic or metabolic, and the two mechanisms may be interrelated. In view of the absence of rash, eosinophilia, and other features of druginduced hypersensitivity, the frequently noted transient elevated liver function tests with gemcitabine therapy in previously published case reports, metabolic idiosyncratic reaction to gemcitabine seems the most compatible mechanistic route of hepatic injury. Classification based on clinical, laboratory, and histological findings are more commonly used, and other patient’s liver disease can be categorized as a cholestatic hepatitis. In our case, radiological findings preceded the liver enzyme abnormality. Sessa et al. [24] showed that gemcitabine induced hepatic toxicity can be increased in the presence of liver metastasis. In a case, reported by Robinson et al., [14], biopsy showed liver fibrosis but autopsy showed capsular and intrahepatic metastasis. It is still not clear why metastasis in liver can increase the risk of gemcitabine toxicity and in a study done by Venook et al. [25], investigating the toxicity of gemcitabine in liver and renal failure, investigators found that gemcitabine toxicity is increased when bilirubin is above 2 mg/dL, however our case shows that gemcitabine induced liver toxicity and fibrosis can be detected by MRI and discontinuation of gemcitabine can prevent further fibrosis and more toxicity. However, without a confirmatory biopsy these changes should be considered as suggestive rather than definitive of the diagnosis of liver fibrosis.
In summary, although gemcitabine has a relatively safe profile but case reports have shown that it can cause serious liver damage. By exclusion, gemcitabine is the probable causative agent in this case, although it can never be completely excluded that a combined effect of gemcitabine together with other medications or factors could be responsible. Gemcitabine should be included in the differential diagnosis of cholestasis during chemotherapy with this agent. MRI can be a noninvasive imaging technique of choice for early detection of gemcitabine induced liver fibrosis and discontinuation of gemcitabine can lead to stabilization of liver fibrosis.Conflict of interest
The authors have no potential conflicts of interest