Original Article - (2017) Volume 18, Issue 4
1Yale-Waterbury Internal Medicine Residency Program, Waterbury Hospital, Waterbury, CT
2Department of Radiology, UCLA Medical Center, Santa Monica, CA
3Department of Pathology, Hannibal Regional Hospital, Hannibal, MO
4Division of Digestive Diseases, University of California, Los Angeles, CA
5John Burns Medical School, University of Hawaii, Honolulu, HI
6UCLA Health Oncology/Hematology, University of California, Los Angeles, CA
7Yale Center for Pancreatic Disease, Yale University, New Haven, CT
Received February 16th, 2017 - Accepted July 07th, 2017
Context In 2011, an international symposium on Autoimmune Pancreatitis produced the International Consensus Diagnostic Criteria, which can be used to stratify patients with autoimmune pancreatitis as having type 1, type 2, or autoimmune pancreatitis – not otherwise specified. There are few studies examining the application of International Consensus Diagnostic Criteria to a cohort of North American patients with autoimmune pancreatitis. Objectives To apply International Consensus Diagnostic Criteria to a cohort of 51 patients with autoimmune pancreatitis followed at a North American medical center. To compare International Consensus Diagnostic Criteria with other guidelines with emphasis on patients who were unclassifiable using International Consensus Diagnostic Criteria. Design We applied International Consensus Diagnostic Criteria using clinical-radiological-pathological features. We reevaluated patients who were unclassifiable per ICDC with Japanese Pancreatic Society-2006, HISORt, Korean, Asian, and JPS-2011 guidelines. We statistically compared type 1, type 2, and unclassifiable patients based on demographic and clinical presentation. T-test and chi-square analysis was used for statistical analysis. Results 37 patients were categorized as definitive type 1 or type 2 autoimmune pancreatitis, 1 patient as probable type 1 autoimmune pancreatitis, and 13 were unclassifiable. Unclassifiable patients had indeterminate/atypical parenchymal imaging or none at all, and 6 patients had elevated serology. Diagnostic endoscopic retrograde cholangio-pancreatography was performed on 6 patients and 1 patient had persistent waxing and waning of clinical and radiologic features. 6 patients could be diagnosed with autoimmune pancreatitis using JPS-2006, Korean, or Asian Criteria, and 4 patients using either HISORt or JPS-2011. There was no statistically significant difference between classifiable and unclassifiable patients based on demographics or clinical presentation. Conclusions The ICDC’s dependence on histology, diagnostic endoscopic retrograde cholangio-pancreatography, and lack of acknowledgment of waxing-waning features limits applicability. Our cohort evolved during routine practice and we identify discrepancies amongst guidelines.
Guideline; Pancreatitis
AIP autoimmune pancreatitis; ERCP endoscopic retrograde cholangio-pancreatography; ICDC International Consensus Diagnostic Criteria; JPS Japanese Pancreatic Society
Autoimmune Pancreatitis (AIP) is a form of chronic pancreatitis that often is difficult to distinguish from malignancy of the pancreas. Unlike pancreatic malignancies however, AIP may respond to therapy with corticosteroids, and has a strong association with other immune mediated diseases and increased levels of immunoglobulin subclass 4 (IgG-4) [1]. AIP has been further classified as either type 1 or type 2 by the International Consensus Diagnostic Criteria (ICDC), primarily by histological features [2].
Although AIP is primarily a pathologic diagnosis, attempts have been made to clinically diagnose AIP using criteria including the Japanese Pancreas Society (JPS-2006 [3], JPS-2011 [4]), Korean Criteria [5], Asian Criteria [6], and Histology Imaging & Serology Other organ involvement and Response to therapy (HISORt) [7]. The ICDC [2] represents a consensus set of criteria produced by a multinational group that could be used in both clinical and research practice. The ICDC [2] describes five cardinal features of AIP including (1) pancreatic imaging of the parenchyma (P) with computerized tomography scan/magnetic resonance imaging (CT/MRI) or pancreatic ductal imaging (D) with endoscopic retrograde cholangiopancreatography/ Magnetic resonance cholangiopancreatography (ERCP/ MRCP), (2) serology (S) with (serum IgG-4 levels), (3) other organ involvement (OOI), (4) histology of the pancreas (H), and (5) response to corticosteroid therapy (Rt).
Unlike older criteria (JPS-2006 [3], Korean Criteria [5], Asian Criteria [6], HISORt [7], ICDC [2] does not require typical pancreatic imaging (CT/MRI/ERCP/MRCP) in order to make the diagnosis of AIP. Instead, multiple avenues can be taken to make the diagnosis, depending on available histology, response to corticosteroid therapy, or pancreatic imaging. Typically, parenchymal imaging is reviewed and categorized as either typical (level 1) or atypical (level 2) for AIP. Depending on which level of evidence is present, the requirements for supporting data vary.
The real world clinical utility of ICDC [2] remains unclear, especially when analyzing patient information retrospectively, as all the clinical-radiological components necessary to confirm a diagnosis may not be available [8]. A recent large multicenter evaluation of the ICDC [2] guidelines favorably validated their use, but used data from clinical centers that were initially involved in the development of the guidelines, possibly contributing to a study bias [9]. A separate validation study that compared Asian [6], HISORt [7], and ICDC [2] guidelines in diagnostic capabilities however concluded that these guidelines should not be used as the gold standard in diagnosis of AIP [10].
In our independent validation study, we aim to apply the criteria set forth by the ICDC [2] to a cohort of patients with AIP from a single institution who had been diagnosed and managed as having AIP in routine clinical practice during the decade leading up to the implementation of ICDC [2] guidelines. We aim to identify the accuracy, ease of application, and potential shortcomings of the ICDC [2]. For comparison, this same patient cohort is also evaluated using other diagnostic criteria (JPS 2006 [3], Korean Criteria [5], Asian Criteria [6], HISORt [7], and JPS 2011 [4]) to identify factors that may contribute to increased accuracy for the diagnosis of AIP, with focus on patients who were unclassifiable per ICDC [2] guidelines.
We enrolled 51 patients who were evaluated and treated with the clinical diagnosis of AIP, between 2001 and 2012 at a single institution Pancreas Clinic prior to the development and publication of the ICDC guidelines. AIP diagnosis was made based on a combination of clinical features including obstructive jaundice and abdominal pain, and where available, serological evidence including increased levels of IgG-4, radiology with CT or MRI findings demonstrating diffuse or focal enlargement of the pancreas, and histological specimens (both surgical resection and pancreatic biopsy), as per the evolving understanding of this disease during this time period. Enrollment in the AIP cohort was made based on a combination of clinical evaluation, histology, and also included multidisciplinary review with gastroenterology, radiology, and surgery subspecialties. While acknowledging the presence of incomplete clinical and investigative studies available during each patient’s workup, we then evaluated each patient who was enrolled in this cohort using the ICDC guidelines. Using the ICDC, our cohort of patients was diagnosed and categorized with as either definitive type 1 AIP, probable type 1 AIP, definitive type 2 AIP, or unclassifiable. We sought to apply the ICDC criteria to our cohort in the least invasive method, preferring to use parenchymal imaging and serology where possible to make the diagnosis. Radiology films were formally rereviewed by a team of radiologists who read each film as a group, allowing for discussion of each radiographic finding and whether or not it met diagnostic criteria for AIP [11].
The patients who were unclassifiable according to ICDC were then identified. Clinical and investigative data available for each of these unclassifiable patients was assessed to see if the inability to confirm the diagnosis of AIP was related to unavailable information. These unclassifiable patients were then re-evaluated using other diagnostic guidelines, to identify clinical-radiological factors which allowed the diagnosis to be made with certain criteria but not others.
We compared type 1, type 2, and unclassifiable patients based on demographics (age, gender), clinical presentation (obstructive jaundice, acute pancreatitis, or any combination) and radiologic imaging (focal or diffuse pancreatic involvement). T-test and chi-square analysis was used with a p-value less than 0.05 being significant (SPSS 20, SPSS Inc, Chicago, IL). This study was approved by the local Institutional Review Board.
Up to the time of publication of the ICDC guidelines we enrolled 51 patients with a working diagnosis of AIP, including 30 men and 21 women, with a mean age of 55.9 and range of 8-81 years (Table 1). After applying the ICDC guidelines to this cohort we obtained the following results.

AIP Definitive Type 1 (Table 2 and Figure 1), from our cohort of 51 patients, 33 patients (64.7%) were diagnosed with definitive type 1 AIP. All 33 patients had level 1 pancreatic parenchymal imaging on CT; defined by the ICDC as diffuse enlargement with delayed enhancement sometimes associated with rim-like enhancement, or presence of low-density mass within the pancreas. With level 1 parenchymal imaging, patients require one other type of evidence (either level 1 or level 2) from any of the following: serology, histology, or other organ involvement. Of the 33 patients, 28 were diagnosed with definitive type 1 AIP with the addition of serological evidence, with 5 patients meeting level 1 serology (>2x upper limit of normal value for serum IgG-4) and 23 patients meeting level 2 serology (1-2x upper limit of normal value for serum IgG-4). Of these 28 patients with level 1 parenchymal imaging and serological evidence, 10 patients also met OOI criteria, and 12 patients had level 1 histology to confirm their diagnosis of definitive type 1 AIP.
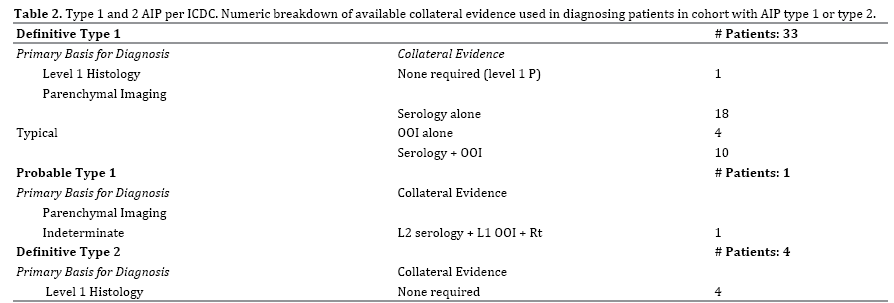
Four patients with level 1 pancreatic parenchymal imaging had other organ involvement (OOI) alone to meet diagnostic criteria. Other organ involvement includes evidence based on radiology or histology. Three of the four met level 1 evidence with radiological evidence: segmental/multiple proximal (biliary/intrahepatic) bile duct stricture, and the remaining one patient with level 2 radiological evidence: symmetrically enlarged salivary/ lacrimal glands. One patient was diagnosed with definitive type 1 AIP with level 1 histology alone. The features met by this patient on pancreatic core biopsy were periductal lymphoplasmacytic infiltration without granulocytic infiltration, obliterative phlebitis, and storiform fibrosis.
AIP Probable Type 1 (Table 2), one patient from our cohort met ICDC diagnosis of probable type 1 AIP. To be diagnosed with probable type 1 AIP a patient with indeterminate/level 2 parenchymal imaging requires a measurable response to steroids, defined as definite improvement in imaging abnormalities or decrease in Cancer Antigen 19-9 (CA 19-9 levels) plus level 2 evidence from either serology, OOI, or histology. Our patient had level 2 parenchymal imaging (segmental/focal enlargement of head and body of pancreas); along with a response to steroids (rapid radiologically demonstrable resolution and marked improvement in pancreatic/extrapancreatic manifestations) plus level 2 serology. This same patient also had level 1 other organ involvement (typical radiological evidence: segmental/multiple proximal hilar/ intrahepatic bile duct stricture).
AIP Definite Type 2 (Table 2), ICDC diagnosed 4 patients from our cohort with definitive type 2 AIP. Unlike type 1 AIP, the diagnosis of type 2 AIP using ICDC requires histological confirmation. All 4 patients demonstrated level 1 histology confirmed IDCP (granulocytic infiltration of duct wall with or without granulocytic acinar inflammation plus absent or scant IgG-4-positive cells).
Clinical Features of Unclassifiable Patients by ICDC
From the original cohort of 51 patients, 13 patients (25.4%) were unclassifiable using ICDC. Of the 13 patients, 6 (46%) presented initially with obstructive jaundice, 5 (38%) with acute abdominal pain and biochemical pancreatitis without obvious risk factors for pancreatitis (such as gallstones or alcohol), and the remaining 2 (15%) presented with pancreatic mass (Table 3). After malignancy was ruled out in these patients on initial evaluation and long-term follow up, diagnosis of AIP was considered.
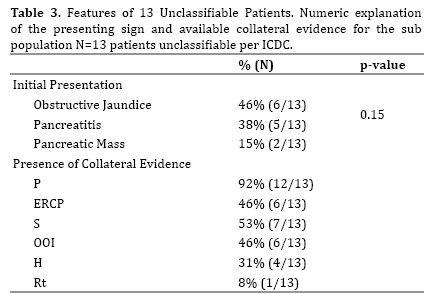
Parenchymal imaging was collected in 12 (92%) patients (level 1: 1 patient, level 2/indeterminate: 10 patients, normal: 1 patient). ERCP was completed in 6 (46%) patients (level 1: 3 patients, level 2: 2 patients, neither level: 1 patient). Serology was available in 7 (54%) patients (level 1: 1 patient, level 2: 5 patients, normal: 1 patient). OOI was investigated in 6 (46%) patients, with 5 meeting criteria based on radiological features (level 1: 4 patients, level 2: 1 patient, neither: 1 patient). A corticosteroid trial was completed in 1 (7%) patient with documented improvement in parenchymal imaging. Histology was available in 4 (30.7%) patients (level 2: 3 patients, neither level: 1 patient) (Table 3).
When the 13 unclassifiable patients were examined using other diagnostic criteria, 6 (46%) patients could be diagnosed with AIP by one or more of the non-ICDC diagnostic criteria. JPS-2006 [3], Korean [5], and Asian [6] guidelines classified the same 6 patients (46%) with AIP. These three guidelines diagnosed patients with a combination of imaging with serology or histology. The HISORt [7] guidelines diagnosed 4 patients (31%) who were unclassifiable per ICDC [2] by utilizing histological features in 3 patients and combination of imaging and serology in 1 patient. JPS-2011 [4] was able to diagnose 4 patients (31%) from the unclassifiable subset (Table 4). In all 4 patients ERCP was paired with serology, OOI, or histology.

Histology amongst Unclassifiable Patients
From our unclassifiable patients, 4 had histology data. These 4 patients were initially evaluated for pancreatic malignancy given their initial clinical presentations of obstructive jaundice, pancreatic mass, or acute pancreatitis. Based on the 5 cardinal features outlined by the ICDC [2], each of these 4 patients demonstrated features suggestive of AIP.
Analysis of ICDC Unclassifiable Patients
Amongst unclassifiable patients Figure 2, we aimed to identify whether patients did not meet ICDC [2] diagnosis due to lack of available data or whether they had available evidence and were still unable to meet ICDC [2] requirements. We looked at the 13 unclassifiable patients and compared the diagnostic ability of other criteria (Table 4).
Using JPS-2006 [3], Korean [5], and Asian [6] guidelines, 6 of the unclassifiable patients (46%) could be diagnosed with AIP. Combination of imaging (parenchymal or ductal) plus serology was utilized to diagnose 4 of these patients. Serology in these 4 patients met level 2 criteria (1 - 2x the upper limit of normal IgG-4 level). The 2 remaining patients could be diagnosed with combination of imaging plus histology (periductal lymphoplasmacytic infiltration). Histology is not differentiated between level 1 and level 2 according to these guidelines, so presence of lymphoplasmacytic sclerosing pancreatitis (LPSP) without regard to additional features mentioned by ICDC [2] (obliterative phlebitis, storiform fibrosis, or presence of IgG-4 positive cells) fulfills histological requirement.
The HISORt [7] guidelines diagnosed 4 patients (31%) who were unclassifiable per ICDC [2]. Histology alone was sufficient to diagnose 3 patients (periductal lymphoplasmacytic infiltration without granulocytic infiltrate). The remaining 1 patient was diagnosed with combination of typical ductal imaging (long stricture of the main pancreatic duct without upstream dilatation) plus level 2 serology (IgG-4 level 1-2x upper limit normal). The reason this patient did not meet ICDC [2] diagnosis is due to indeterminate parenchymal imaging according to ICDC [2] with absence of sufficient supporting evidence.
While JPS-2011 [4] could diagnose 4 patients in our cohort that ICDC [2] could not, 3 of those cases (75%) necessitated ERCP as JPS-2011 [4] requires ERCP in all patients with level 2 parenchymal imaging. The requirement of ERCP in cases of indeterminate parenchymal imaging is to avoid misdiagnosis of pancreatic cancer [4]. In these 3 patients, ERCP findings were paired with combination of serology, OOI, or histological features to diagnose AIP. The remaining 1 patient had no parenchymal imaging available, however had typical ERCP findings for AIP along with level 2 histological features (periductal lymphoplasmacytic infiltrate without granulocytic infiltration plus storiform fibrosis). 7 patients (13.7%) were unable to be diagnosed with AIP by any of the discussed criteria due to unavailable data.
Statistical Analysis
There was no statistically significant difference between both classifiable and unclassifiable patients in terms of patient demographics, clinical presentation and radiologic imaging. However, all patients who presented with combination of acute pancreatitis, obstructive jaundice, and pancreatic mass were diagnosed with AIP per ICDC [12, 13].
We present a retrospective validation study for the ICDC [2] guidelines using a prospectively collected cohort of 51 North American patients followed clinically from 2000 - 2012 prior to the publication of ICDC [2]. Our patient cohort has a similar age range and gender distribution at diagnosis (56.4 years and 61.5 years for type 1 and type 2, respectively) (Table 1) when compared to other previously published large studies [9, 14]. A previously published study that examined a North American cohort reported a slightly older average age on presentation of 63 years for type 1 patients, with majority (83%) being male [15].
A suggested benefit of the ICDC [2] guidelines is the flexibility allowed in the diagnostic evaluation of patients; the diagnosis of AIP can be made through a variety of pathways. For example, typical pancreatic parenchymal imaging can be paired with data from serology, radiology, histology, or response to steroid therapy [16]. In the event parenchymal imaging is atypical or indeterminate, the diagnosis of AIP is still possible with a separate combination of data. This is important because patient presentation is not uniform and diagnostic criteria that allow for flexibility are favorable in clinical practice, especially if designed for use in primary care settings. Another advantage of ICDC [2] is that unlike JPS-2011 [4], ICDC [2] does not necessitate ERCP in cases with atypical parenchymal imaging, further supporting the use of ICDC [2] amongst primary care physicians. Though not required, ductal imaging can serve a complementary role in the diagnosis if available. However, as seen in our cohort, ERCP did not assist in diagnosis of any patient in our cohort. ICDC [2] also recognizes AIP as the pancreatic manifestation of IgG-4-related disease and as such recognizes the other organs associated with IgG-4-related disease as supportive evidence for diagnosing AIP, such as biliary strictures, retroperitoneal fibrosis, and sialadenitis. IgG-4-related disease can involve nearly every organ system, and as such recognition of AIP requires awareness of the possible array of other organ involvement [17].
While several prior studies have found that ICDC [2] had superior diagnostic sensitivity, this current study is not as supportive of the utility and diagnostic capability of ICDC [2]. In one such study, five major diagnostic guidelines (ICDC [2], Korean [5], Japanese-2011 [4], Asian [6], and HISORt [7]) were evaluated using a Japanese cohort of patients with AIP, revealing that ICDC [2] had the greatest sensitivity [18]. Similarly, a Taiwanese [19] cohort was analyzed using ICDC [2], HISORt [7], and Asian [6] criteria, and again ICDC [2] was found to have superior sensitivity. In a third study involving an Japanese cohort of 110 Japanese patients with AIP treated between 1992 and 2013, ICDC [2] demonstrated superior accuracy (95% diagnosis) compared to JPS 2006 [3], Asian [6], HISORt [7], ICDC [2], and revised JPS 2011 criteria [4]. While ICDC [2] had the highest accuracy for diagnosis, they concluded that ICDC [2] was intended for experts of pancreatology and JPS-2011 [4] on the other hand was more compatible for general internists. The reasons cited against ICDC [2] included the disregard for country specific diagnostic criteria, given ICDC [2] was meant to be used globally. Also, the use of different levels (level 1 and level 2) of evidence, may result in complicating the process of diagnosis [4].
A North American cohort at a large U.S. center evaluated 26 patients with AIP and reported that diagnostic guidelines including HISORt [7], JPS 2006 [3], and Korean [5] guidelines were satisfied for the diagnosis of AIP in 85% of cases, suggesting that various guidelines serve complementary roles [20]. This study did not compare the guidelines for superiority in diagnostic capabilities. Similarly in a Dutch [10] study, 114 patients with AIP were re evaluated using Asian [6], HISORt [7], and ICDC [2] guidelines. In this study, 82% satisfactorily met at least one of these guidelines for diagnosis of AIP suggesting a complementary role amongst guidelines. The remaining 18% unable to be diagnosed using any of the three diagnostic guidelines. In another Italian study [21], 92 patients with AIP were re-evaluated with ICDC [2]. Of the 92 patients, 15 patients (16%) were classified as not otherwise specified. This study further concluded that patients who were deemed not otherwise specified were classified as such despite having many features that were similar to patients classified as type 1 or type 2 AIP.
To our knowledge our study is the only North American study to evaluate a cohort of patients with clinically diagnosed AIP with application of multiple guidelines including ICDC [2]. Unlike the international studies discussed above [4, 18, 19], ICDC [2] sensitivity (74.5%) was not superior (38/51 patients with either type 1 or type 2 AIP) relative to clinical diagnosis, which served as the gold standard. A significant barrier to diagnosis using ICDC [2] guidelines amongst our cohort was the distinction between level 1 and level 2 evidence. We often encountered patients who clinically were diagnosed and treated as having AIP, however could not be classified as having level 1 histology or other organ involvement due to the absence of a single feature. This detail also explains why JPS 2006 [3], Korean [5], and Asian [6] guidelines could each diagnose a larger proportion of our cohort, as these guidelines do not distinguish between level 1 and level 2 evidence. When using all guidelines together, 44 patients (86%) could be diagnosed with AIP, suggesting that using multiple guidelines can serve a complementary function.
Ductal imaging is not required for the diagnosis of AIP per ICDC [2] guidelines. Instead, ERCP can be used as supportive evidence, especially when parenchymal imaging is indeterminate. Though not mandatory, ductal imaging can help to differentiate between AIP and pancreatic cancer. In a comparison of ERCP features amongst AIP and pancreatic cancer patients, it was noted that obstruction of the main pancreatic duct and skipped main pancreatic duct lesions were far more typical of AIP than pancreatic cancer [22]. However, if parenchymal imaging is highly suggestive of AIP then ductal imaging may not be necessary at all. Amongst our cohort, 13 patients had parenchymal imaging meeting indeterminate evidence for AIP, of which 8 underwent ERCP. Of these 8 patients, 3 demonstrated significant strictures of the main pancreatic duct, which is highly suggestive of AIP per multiple studies [2, 22]. The inclusion of ERCP in the ICDC guidelines is unnecessary as seen in our cohort, as all patients could be diagnosed without ERCP. From our cohort of 51 patients with AIP, 34 (67%) patients underwent ERCP as part of their evaluation and the ERCP findings in these patients did not contribute to diagnosis in any patient. Furthermore, diagnostic ERCP is used less frequently in North America and the justification for performing pancreatic ductal imaging in the evaluation of AIP is not clear in our cohort.
Histology is required in diagnosing type 2 AIP. Several methods for obtaining tissue in evaluation of pancreatic masses are available, including ERCP with papillary biopsy; CT guided percutaneous core biopsy, and endoscopic ultrasound (EUS) with fine needle aspiration (FNA), all of which carry favorable safety and diagnostic profiles [23, 24]. However, the diagnostic utility and indications for performing these studies remain unclear in evaluation of patients with CT/MRI findings suggestive of AIP. Thus unnecessary procedures are performed. In one European study evaluating 29 patients with ICDC [2] diagnosed AIP, it was noted that a total of 50 ERCP and 18 EUS procedures were performed, with retrospective analysis concluding that only 20 ERCPs and 4 EUS procedures could be justified. Additionally, 8 patients (23%) were referred to surgery that could not be justified [25].
EUS with FNA has been evaluated in several cohorts of patients who had CT or MRI imaging findings suggestive of AIP. In one such study, tissue collected using EUS with FNA diagnosed 45 out of 78 patients (57.7%) with AIP using ICDC guidelines [26]. There is disagreement however, with additional studies either supporting or refuting the use of EUS with FNA as an effective diagnostic tool, citing favorable and unfavorable sensitivities [27, 28].
Histology, though mandatory for diagnosing type 2 AIP, provided no additional supportive data in our type 1 patients. Amongst type 1 patients, 27 of 34 patients (79%) had histology available, with 20 cases yielding evidence (level 1: 13 patients, level 2: 7 patients). Amongst unclassifiable patients, 4 out of 13 patients (31%) had histology collected, with 3 patients yielding level 2 evidence. However, regardless of level 2 histology findings, these patients remain unclassifiable per ICDC [2].
We believe that diagnostic guidelines including ICDC [2] should acknowledge that spontaneous relapsing and remitting symptoms or imaging features without steroid therapy are characteristic of an inflammatory etiology and favor the diagnosis of AIP over pancreatic malignancy. AIP has been linked with high serum level of immune complexes which favors the idea that complement activation is involved at least during relapses [29]. Lack of consideration of patients who show spontaneous improvement in symptoms, imaging, or serology without corticosteroid therapy became evident amongst our cohort when we noted 1 patient who had this distinct feature. This patient, who presented with obstructive jaundice and pancreatic mass, was found to have typical parenchymal imaging for AIP, level 1 OOI, and benign histology. Clinically this patient was diagnosed and treated as AIP. However, the patient showed spontaneous resolution in imaging and symptoms without corticosteroid therapy, which lends additional support against this patient having a malignant process and instead more likely having an inflammatory etiology. Had this patient been given steroids it may have become difficult to distinguish whether remission was spontaneous or corticosteroid induced.
There are several limitations to our current study. Our study data was acquired during the clinical management of each patient in the absence of the ICDC [2] guidelines. Patients were diagnosed and treated with AIP based on clinical evidence and without the utilization of ICDC [2], and was thus free of selection bias. Given that our study was retrospective, we utilized only the data that had been collected during the course of clinical evaluation, which may have been incomplete when applying the ICDC [2] guidelines. For example, while IgG-4 levels were collected in the majority of patients, they were not available for 11 patients (21.5%). The role of IgG-4 serology in patients with AIP has been outlined to illustrate patients with AIP have on average higher levels of serum IgG-4 compared to unaffected patients [1]. Additional data not available in all patients were ERCP and steroid trials, in 17 and 38 patients, respectively. With more data points available, a more critical evaluation could be made regarding the ICDC [2] guidelines. This limits the proper evaluation of each cardinal feature of its contribution of the diagnostic procedure in this cohort.
Another limitation of this type of study is the absence of a gold standard pathologic diagnosis for all patients. As such it is possible that some of our patients who were unclassifiable using ICDC [2] may not have even had AIP, even if more diagnostic data were available. While several studies evaluating various diagnostic criteria have shown that ICDC [2] has superior sensitivity [18, 19], up to 20% of patients with AIP can be unclassified by any diagnostic criteria [10].
While the ICDC represents consensus understanding and recommendations for diagnosing AIP, application in our cohort provides an alternative impression [4, 18, 19]. We find that several features should be refined moving forward including acknowledging that patients with AIP can have waxing and waning of symptoms and clinical features, identifying suitable justifications and indications for invasive testing such as ERCP, and validation of methods used for acquiring histologic data.
Furthermore, from our study it appears that utilizing multiple diagnostic guidelines in complementary roles can lead to more accurate diagnosis in patients with suspected AIP. Future directions for study include comparison of methods used to acquire histology and evaluation of the utility of ERCP inclusion in the guidelines.
The authors declare that they have no conflict of interest.