- (2010) Volume 11, Issue 6
Kofi Oppong1, Dan Raine1, Manu Nayar1, Viney Wadehra2, Subramaniam Ramakrishnan3, Richard M Charnley4
Departments of 1Gastroenterology and 4HPB Surgery, Freeman Hospital, Newcastle upon Tyne, United Kingdom.
2Department of Cytopathology, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom.
3Department of Gastroenterology, Warrington Hospital, Warrington, United Kingdom
Received June 15th, 2010 - Accepted August 11th, 2010
Context Individuals with suspected malignant biliary obstruction commonly undergo ERCP for drainage and tissue sampling via biliary brushings. EUS with EUS-FNA facilitates staging and potentially more accurate tissue sampling. Objective The aim is to compare the diagnostic performance of EUS-FNA and ERCP with biliary brushings (ERCP-BB) in the diagnosis of pancreatobiliary carcinoma and the utility of combining the two procedures under conscious sedation. Design Retrospective analysis of a prospectively maintained database. Patients Thirty-seven patients with suspected malignant obstructive jaundice underwent 39 paired procedures, either combined (n=22) or within a few days (n=17). Results Using strict cytological criteria the sensitivity of EUS-FNA in the diagnosis of malignancy was 52.9% (95% CI: 35.1-70.2%) versus 29.4% (95% CI: 15.1-47.5%) for ERCP-BB. Combining the two tests improved sensitivity to 64.7% (95% CI: 46.5-80.3%) which was significantly better than ERCP-BB alone (P=0.001) but not EUS-FNA alone (P=0.125). When both procedures were performed under the same conscious sedation, there was a significant difference (P=0.031) between the sensitivity of EUS-FNA (52.6%; 95% CI: 28.9-75.6%) and that of ERCP-BB (21.1%; 95% CI: 6.1-45.6%). When both procedures were performed together the mean±SD in-room time was 79±14 min (range: 45-105 min). Two of the patients (9.1%) had a complication. Conclusions In patients undergoing EUS-FNA and ERCP-BB under the same sedation, EUS-FNA was significantly more sensitive in diagnosing malignancy. Combining the results of both tests improved diagnostic accuracy. Combining therapeutic ERCP and EUS-FNA under the same conscious sedation is feasible, with a complication rate similar to that of ERCP alone.
Carcinoma, Pancreatic Ductal; Cholangiopancreatography, Endoscopic Retrograde; Endosonography
ERCP-BB: ERCP with biliary brush cytology
The majority of patients presenting with signs and symptoms of malignant biliary obstruction are ultimately not suitable for surgery, either due to locally invasive or metastatic disease, or because of comorbidities. Only 10-15% of pancreatic cancers [1] and 20-49% of cholangiocarcinomas are resectable [2, 3]. For inoperable patients therapy with either chemotherapy or radiotherapy requires a definitive pathological diagnosis. In addition, the quality of information provided to the patient regarding prognosis is severely compromised if the exact diagnosis is unknown.
Prior to the development of endoscopic ultrasonography (EUS), ERCP with biliary brush cytology (ERCP-BB) was the initial investigation of choice for cytopathological diagnosis in jaundiced patients with suspected pancreaticobiliary malignancy. Although this technique has a specificity approaching 100%, sensitivity for malignancy has been reported as 15-65% in strictures secondary to pancreatic cancer and 44- 80% in strictures due to cholangiocarcinomas [4, 5]. Overall sensitivity is the order of 42% [5].
EUS allows excellent visualisation of the pancreas and adjacent organs and has evolved as a sensitive staging modality for pancreatobiliary malignancy [6, 7, 8]. The addition of EUS-guided fine needle aspiration (EUSFNA) allows cytological diagnosis of pancreatic masses. This has been shown, in many published series, to be highly accurate in diagnosing pancreatic masses [9, 10, 11]. In a retrospective multicenter study EUS-FNA was diagnostic of malignancy in 71% of solid pancreatic masses [12]. This conveys a significant advantage over traditional ERCP-based cytology.
The ERCP-BB and EUS-FNA data however may not be directly comparable.
In contrast to studies relating to biliary brush cytology, the majority of published series of EUS-FNA with high sensitivities have had a cytopathologist or cytopathology technician present in the room [9], allowing multiple sampling to occur until adequate tissue has been aspirated. Increasingly, non-operable patients will have both an ERCP for palliation and biliary brushings, and where available an EUS for staging and FNA often on separate occasions.
In this study, we compare these two diagnostic modalities in a population of jaundiced patients referred to a tertiary referral centre in which the clinical suspicion of malignant obstruction was high. In addition the utility of combining the two procedures under the same (conscious) sedation was assessed.
As in-room cytology was not available in our unit during the study period. All patients having either ERCP or EUS within a few days of each other requiring a tissue diagnosis underwent both biliary brushings and FNA. Also, in order to expedite the investigation of patients requiring both ERCP (for biliary drainage and brush cytology) and EUS for staging and FNA, we sought to combine the procedures under the same sedation whenever possible. All procedures were performed for clinical indication and not to research protocol. Clinical management decisions were made through our multi-disciplinary team process. All patients had a thin-slice multidetector pancreatic protocol CT study prior to endoscopic intervention.
ERCP and EUS data was recorded prospectively as part of our ongoing quality monitoring. Retrospective review was also performed of clinical case notes and electronic patient record. All patients in whom ERCPBB and EUS-FNA were performed sequentially under the same sedation and those in whom the second procedure was performed prior to availability of the results of the first sampling procedure were identified. A total of 38 patients (21 male, 17 female; mean age 62.4 years, range 26-87 years) between February 2004 to May 2007 met the above criteria. All the patients presented with obstructive jaundice and had either an indeterminate biliary stricture or a mass in the head of pancreas, with a requirement for biliary drainage and a formal tissue diagnosis for the purpose of planning treatment (surgical or conservative). One patient with a final diagnosis of lymphoma was excluded from further analysis. Therefore, 37 individuals underwent 39 paired procedures (two individuals had two paired procedures); 22 paired procedures were performed sequentially under the same sedation and 17 paired procedures were performed within a few days (17 cases; mean interval: 2 days; range: 1-4 days).
All procedures were performed under conscious sedation (administered by the endoscopist) with midazolam and pethidine.
ERCP and biliary brushings were performed or supervised by either K.O. or R.M.C., both of whom are highly experienced biliary endoscopists (more than 2,000 ERCP) using TJF200, TJF240 and TJF260 duodenoscopes (Olympus UK, Southend on Sea, United Kingdom). All EUS-FNA procedures were performed or supervised by K.O. (more than 500 pancreatic EUS-FNA). When performed simultaneously, ERCP was generally performed first as the priority was to ensure biliary drainage. Brushings were taken using a standard cytology brush (M0054500; Boston Scientific, St Albans, United Kingdom) and standard technique. The brush catheter was advanced over a wire and under fluoroscopic control to the lower margin of the stricture. The brush was then advanced and retracted a minimum of three times, the catheter removed, the brush wiped on a glass slide and the slide and brush tip sent for cytological assessment. EUS and EUS-FNA was performed using an echoendoscope (EG383OUT; Pentax, Slough, United Kingdom) and ultrasound workstation (EUB 6500; Hitachi Medical Systems, Wellingborough, United Kingdom). Wilson Cook Predominantly 22G needles were used, while 25G needles were used on occasion (Cook Ireland Ltd., Limerick, Ireland). A standard technique was used. The mass was identified and after staging assessment and the use of Doppler to assess for vessels, the FNA needle was passed into the lesion under EUS control. Suction was used and the needle moved within the tumour for 6-10 throws. The needle was removed and the stylet replaced to express tissue onto cytology slides, these were air dried and stained when dry with Grunwald Giemsa stain. Needle rinsings were processed as a cytospin using Papanicolaou stain. During the course of the study liquid-based cytology (SurepathTM; Bioscience Healthcare, Nottingham, United Kingdom) was introduced for both FNA and BB cytology and was performed as well as the preparation of conventional slides.
A cytopathologist was not present in the endoscopy suite for any of the procedures. The endoscopy room time (duration from patient entry into the endoscopy room to their exit) was recorded. This time comprises brief preparation and positioning prior to the procedure, the procedure time and then brief repositioning time prior to taking the patient to the recovery area.
The results of the ERCP and EUS procedures were compared with the following reference methods of tissue diagnosis:
1) surgical histology or other biopsy methods (e.g. percutaneous sampling of the primary tumour);
2) any positive cytology result combined with clinical follow-up that provided further evidence of malignancy;
3) clinical, biochemical and radiological follow-up until death or for at least two years if there was no pathological or radiological evidence of malignancy.
Written informed consent was obtained from all patients prior to the procedures. All procedures were done as a part of standard patient care and not to a research protocol and data collection was performed as part of our ongoing clinical audit (quality monitoring). Therefore institutional review body approval was not required. Normal NHS Clinical Audit Practice was observed. All aspects of the study were conducted in accordance with the Declaration of Helsinki 1964, as revised in Tokyo 2004.
Sensitivity, specificity, positive and negative predictive values, and accuracy were determined for ERCP biliary brush cytology, EUS-FNA and a combination of the two diagnostic methods. Diagnostic accuracy was defined as the frequency of cases correctly classified. The exact 95% confidence intervals (95% CI) of frequencies were calculated by means of the binomial distribution [13]. Differences in diagnostic sensitivity and accuracy between pairs of the three methods were identified by using the McNemar test and the statistical significance was assessed at the 0.05 level (two-tailed). Statistical analysis was performed using SISA [14] and Medcalc (version 11.3.1.0; https://www.medcalc.be/).
The mean bilirubin was 250 μmol/L (reference range: 0-19 μmol/L). All patients had a final definitive diagnosis achieved (Table 1). In 13 patients this was by surgery, 17 patients had diagnostic cytology and underwent no further intervention, 3 patients had subsequent diagnostic histology or cytology and 4 patients had clinical follow-up until death (n=2) or for greater than two years establishing benign disease (n=2).
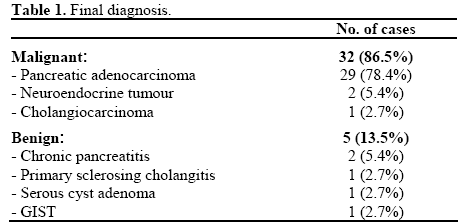
Of the 37 patients, 32 (86.5%) had malignancy; pancreatic adenocarcinoma (n=29), neuroendocrine carcinoma (n=2) and cholangiocarcinoma (n=1). Five patients (13.5%) had a final diagnosis of benign disease; chronic pancreatitis (n=2), primary sclerosing cholangitis (n=1), serous cystadenoma (n=1) and GIST (n=1). The two individuals who had two paired procedures had malignancies.
The mean bilirubin was 250 μmol/L (reference range: 0-19 μmol/L). All patients had a final definitive diagnosis achieved (Table 1). In 13 patients this was by surgery, 17 patients had diagnostic cytology and underwent no further intervention, 3 patients had subsequent diagnostic histology or cytology and 4 patients had clinical follow-up until death (n=2) or for greater than two years establishing benign disease (n=2).
Of the 37 patients, 32 (86.5%) had malignancy; pancreatic adenocarcinoma (n=29), neuroendocrine carcinoma (n=2) and cholangiocarcinoma (n=1). Five patients (13.5%) had a final diagnosis of benign disease; chronic pancreatitis (n=2), primary sclerosing cholangitis (n=1), serous cystadenoma (n=1) and GIST (n=1). The two individuals who had two paired procedures had malignancies.
The mean number of passes was 2.7 (range: 1-6). Of the 39 EUS-FNA performed, 18 (46.2%) were diagnostic for malignancy, 7 (17.9%) were suspicious and 14 (35.9%) negative. All 7 suspicious aspirations were subsequently proven to have malignancy, by surgery (n=5), positive biliary brushings and follow-up (n=1) and clinical follow-up alone (n=1). Of the 14 negative specimens, 5 (35.7%) were proven to be benign by surgery (n=3) and clinical follow-up (n=2), 9 (64.3%) were proven to have malignancy by surgery (n=4), positive repeat FNA (n=3), positive biliary brushings and follow-up (n=1) and clinical follow-up alone (n=1). Accepting diagnostic cytology only EUSFNA had an accuracy of 59.0%, sensitivity of 52.9%, specificity of 100%, a positive predictive value (PPV) of 100% and a negative predictive value (NPV) of 23.8% (Table 2A). Including the 7 suspicious cases gave an accuracy of 76.9%, sensitivity of 73.5%, specificity of 100%, PPV of 100%, and NPV of 35.7% (Table 2B).
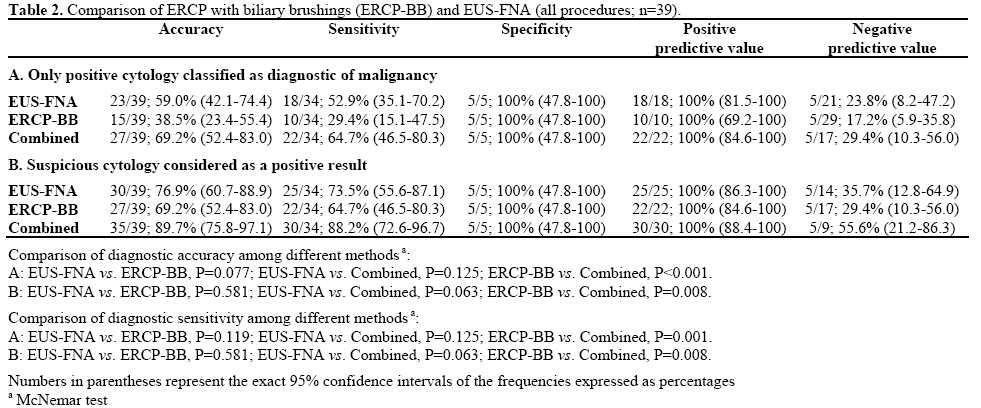
Biliary Brushing
Biliary brushings were obtained in 38 of the 39 cases (in one case it proved impossible to pass a wire across the subsequently proven malignant stricture). On an intention to treat basis this case was treated as a false negative. In 10 cases (25.6%) cytology was positive, in 12 suspicious (30.8%) and in 17 negative (43.6%). All 12 suspicious cases were subsequently proven to have malignancy by surgery (n=4), positive FNA (n=5), positive repeat FNA (n=2) or by clinical follow-up alone (n=1). Of the 17 negative cases, 5 (29.4%) were true negatives by surgery (n=3) and clinical follow-up (n=2) and 12 (70.6%) were false negatives. Accepting only positive cytology as diagnostic of malignancy, the accuracy of biliary brushings was 38.5%, sensitivity 29.4%, specificity 100%, PPV 100% and NPV 17.2% (Table 2A). Including the 12 suspicious cases accuracy was 69.2%, sensitivity 64.7%, specificity 100%, PPV 100%, and NPV 29.4% (Table 2B).
The difference of and between biliary brushings and EUS-FNA was not statistically significant both when only positive cytology as diagnostic of malignancy (accuracy: P=0.077; sensitivity: P=0.119; Table 2A) or including the suspicious cases (accuracy and sensitivity: P=0.581; Table 2B) were considered.
Combination
An analysis was performed to investigate the utility of combining the results of brushings and EUS-FNA. Accepting only positive cytology as diagnostic of malignancy, gave an accuracy of 69.2%, sensitivity of 64.7%, specificity of 100%, PPV of 100%, and NPV of 29.4% (Table 2A). This was significantly more accurate and sensitive (P<0.001 and P=0.001, respectively) than brushings alone but not EUS-FNA alone (P=0.125). Accepting positive and suspicious cytology as diagnostic gave an accuracy of 89.7%, sensitivity of 88.2%, specificity of 100%, PPV 100%, and NPV 55.6% (Table 2B). This was again significantly more accurate and sensitive than biliary brushings alone (P=0.008) but not EUS-FNA alone (P=0.063).
Simultaneous Procedures
Of the 39 procedures, 22 (56.4%) were performed simultaneously. There were 12 female and 8 male patients (1 male and 1 female underwent a dual procedure twice). Mean age was 60.5 years (range: 26- 77 years). One procedure was under general anaesthetic. The other 21 procedures were performed under conscious sedation with pethidine and midazolam administered by the endoscopist.
Tissue Sampling
EUS-FNA was performed in all 22 combined procedures, the mean number of passes being 2.9 with a range of 1-6. Biliary brushings were obtained in 21 procedures. In one case as stated previously it proved impossible to pass a wire across the subsequently proven malignant stricture. On an intention to treat basis this case was treated as a false negative. For EUS-FNA, 10 positive specimens (45.5%) were obtained and 3 suspicious (13.6%). There were 9 negative aspirates (40.9%), 6 of which were false negatives (66.7%). For biliary brushings there were 4 positive samples (18.2%), 6 suspicious (27.3%) and 12 negative samples (54.5%), 9 of which were false negatives (75.0%). Test performance either accepting positive cytology only as diagnostic, or including suspicious samples is shown in Table 3. When only positive cytology was accepted as diagnostic there was a significant difference between ERCP-BB and EUSFNA (accuracy and sensitivity: P=0.031; Table 3A) and the combination of EUS-FNA and ERCP-BB was significantly more accurate and sensitive than ERCPBB alone (P=0.031; Table 3A). No significant differences were observed among the three methods when suspicious cytology was considered as a positive result (Table 3B).
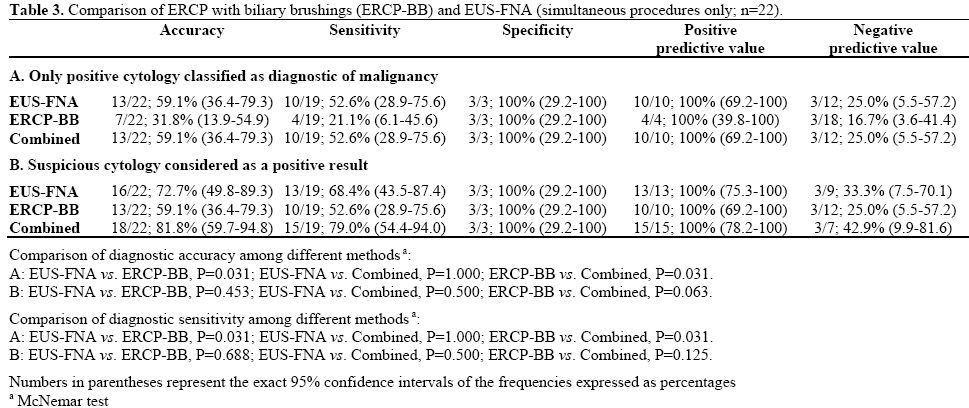
Procedure Duration and Sedation
The mean±SD in-room time for 19 of the 21 (data unavailable for 2 patients) simultaneous procedures under conscious sedation was 79±14 min (range: 45- 105 min). For comparison the mean duration of 44 consecutive EUS-FNA for pancreatic masses during a 3-month period of this study was 45±14 min (range: 15-65 min) and for ERCP (51 consecutive cases) 56±25 min (range: 20-125 min). The average dose of midazolam administered was 8.5 mg (range; 2.0-12.5 mg) and of pethidine 47 mg (range 25-50). No flumazenil was given.
Complications
There were two complications from the simultaneous procedures (9.1%). One patient had a mild attack of pancreatitis (4.5%) and one patient (4.5%) failed to drain and required a stent change a week later.
The pre-test probability of malignancy is high and has been reported as greater than 90% in a patient presenting with a pancreatic mass and jaundice [15]. The differential diagnosis of pancreatic adenocarcinoma includes focal chronic pancreatitis, autoimmune pancreatitis and pancreatic neuroendocrine tumour [16, 17]. An accurate preoperative diagnosis is desirable but is not absolutely necessary because most patients will undergo attempted resection based on the presence of a mass and obstructive jaundice. Insisting on a confirmed diagnosis of malignancy in fit patients with an operable suspicious lesion in the head of the pancreas can delay surgery and allow the tumour to progress. In elderly and comorbid patients, however, confirmation of the diagnosis preoperatively should be sought more keenly since surgery carries a greater risk and is best avoided in those without malignancy. CT is the first investigation of choice in patients with suspected pancreatic cancer. The absence of a mass on CT, however, is an indication for EUS which has an important role in clarifying the diagnosis prior to surgery [6]. In those patients with unresectable disease, chemotherapy is the treatment of choice. In patients with benign disease (other than autoimmune pancreatitis) surgery is often the most appropriate treatment, since symptoms caused by biliary obstruction and duodenal stenosis can be effectively treated. Chemotherapy is contra-indicated in those who may have benign disease and therefore positive histology or cytology is essential in those patients who are to be treated by non-surgical treatments.
Establishing the diagnosis in pancreatic cancer is not easy. The tumour is commonly confined to the pancreas and it is unusual for the tumour to erode an epithelial surface such as stomach or duodenum. A lesion in the head of the pancreas will usually cause obstructive jaundice due to its proximity to the bile duct. Extrinsic compression of the bile duct, however, will cause obstruction even before the tumour erodes onto the biliary epithelium. In this case malignant cells may not be present within the lumen of the bile duct. Prior to the advent of EUS-FNA, biliary brush cytology was the foremost method of obtaining a cytological diagnosis in patients presenting with jaundice and a biliary stricture. EUS-FNA is well documented to achieve high accuracy and sensitivity in the diagnosis of pancreatic masses. EUS-FNA has shown high sensitivity where prior biliary brushings or percutaneous biopsies have been negative or non diagnostic [2, 11]. The prior sampling procedure may have been performed in the referring hospital however, and read by a cytopathologist without particular expertise in pancreatic and biliary cytology. The experience of the cytopathologist has previously been shown to have significant impact on the accuracy of the report [18]. In addition, the majority of EUS-FNA studies reporting high accuracy have utilised an inroom cytopathologist, which has been shown to improve diagnostic yield [19, 20]. This is not available in many units. In a recent large retrospective series [21] EUS-FNA (utilising strict cytological criteria) had an overall sensitivity of 77% in the diagnosis of pancreatic neoplasia. In this study immediate cytological examination was available for 43.8% of procedures and significantly improved test performance. Our study reports the comparison between EUS-FNA and biliary brush cytology (39 procedures) and the utility of sequential procedures under the same sedation (22 procedures). In the subset having simultaneous procedures, ERCP, biliary brushings and stent insertion as well as EUS with EUS-FNA were performed utilising endoscopist-administered conscious sedation. All EUS-FNA was performed by one operator and ERCP by two. Both brush and aspiration cytology were reported by the same experienced pancreatobiliary cytopathologists. A previous study by Rosch et al. [22] has directly compared ERCP and EUS for the tissue diagnosis of biliary strictures. In this study there were 50 paired procedures of which 12 were performed during the same session. No difference was found between ERCP-BB and EUS-FNA in the detection of malignancy although there was a numerical advantage to ERCP-BB in the detection of biliary malignancy and EUS-FNA for pancreatic masses. There was no inroom cytopathologist and it is not stated in the paper as to what sedation was used for the 12 procedures done in one session.
Two other studies have reported on the utility of combining ERCP and EUS-FNA in the same procedure [23, 24]. Both of these studies utilised propofol sedation administered by an anaesthetist. In the study by Ross et al. [23] 87 of the 112 patients had a FNA whilst biliary brushings were obtained in 54. EUSFNA had an overall accuracy of 86.7% and a sensitivity of 83.3%, while brush cytology had a sensitivity of 13% and an accuracy of 48%. In the Ross et al. study [23] EUS-FNA was performed first and brushings only taken if there was a delay in reporting the EUS-FNA cytology or if FNA cytology was not positive and is therefore not a true comparison of the two tests. In a study by Tarantino et al. [24], 72 patients underwent combined ERCP and EUS, EUSFNA being performed on 25 of the patients. It is not reported whether biliary brushings were obtained. An in-room cytopathologist was available for analysis of the FNA in both of these studies. In the study by Ross et al. [23] suspicious aspirates were excluded from analysis and not considered as either diagnostic or false negative. In the Tarantino et al. study [24] the cytological criteria for a diagnostic aspirate is not stated. In our study (using strict cytological classification) the performance of biliary brushings (accuracy 38%) although in line with the reported literature is at the lower end of the range. The performance of EUS-FNA appears at the lower end of the range (accuracy 59%) possibly because of adherence to strict diagnostic cytology criteria and the absence of in-room cytopathology as compared to other studies [9, 23, 24]. If suspicious cytology is considered diagnostic as has been reported in a number of studies [21, 25] the performance characteristics are improved (sensitivity 73%, accuracy 77%) and in line with such studies. Another approach in early studies was to exclude nondiagnostic specimens in addition to classifying suspicious cytology as positive [26]. In our study, if nondiagnostic aspirates are excluded and suspicious cytology considered diagnostic, the sensitivity, negative predictive value, and accuracy of EUS-FNA are 80%, 40% and 83%, respectively.
In the present study we have documented that when performed under the same sedation, by the same cytopathologist, using strict cytological diagnostic criteria and in the absence of in-room cytopathology EUS-FNA is more sensitive than ERCP with biliary brushings in the diagnosis of pancreatobiliary carcinoma. We have also demonstrated that combining the results of the FNA and brushings significantly improves the result compared to ERCP-BB alone and that if suspicious cytology is classified as a positive diagnosis the combined test performance is comparable (accuracy 90%, sensitivity 88%, negative predictive value 56%) to that reported for EUS-FNA in units with in-room cytopathology.
A concern with performing EUS and ERCP during the same session has been the risk of complication [27, 28]. ERCP is associated with a complication rate of 4- 13% [29, 30, 31, 32, 33] with a reported pancreatitis rate that varies from 1% to 13%. [29, 32, 33, 34]. EUSFNA is associated with a lower risk of post-procedure pancreatitis less than 1% [35, 36] and has an overall lower reported complication rate of 2.5-5% [36, 37]. In our series the overall complication rate was 9.1%, all of which were mild, which is comparable to the complication rate reported for ERCP only and the 10.5% complication rate reported by Ross et al. [23] in their study of combined EUS and ERCP. We had one case of pancreatitis (4.5%). We have previously reported a 3.0% post-ERCP pancreatitis rate in our unit [38].
`A potential concern when combining two potentially lengthy procedures under the same sedation particularly using endoscopist administered conscious sedation is of an overly prolonged procedure and excess sedation. The endoscopy room time in this study (79±14 min; range: 45-105 min; 19 procedures) was very similar to the procedure time reported by Ross et al. [23] of 74.6±30 min (range: 25-148 min) and the time of 58.6±16.4 min (range: 30-91 min) in the study by Tarantino et al. [24]. In contrast to these previous studies all but one of the 22 simultaneous procedures in our series were performed under conscious sedation. The mean midazolam dose was 8.5±3.7 mg, this is significantly more (P=0.00017) than the mean dose of 6 mg that we documented in an audit of 482 ERCP procedures from our institution [38]. Concerns have been raised about prolonged sedation during ERCP in the elderly [39]. In this small series there was no complication attributable to sedation. We however feel that the future of such complex endoscopy lies in deeper sedative techniques allied to more intensive monitoring [40].
As a retrospective review from one unit there are limitations to this study; it did not include consecutive patients requiring ERCP and EUS, the numbers are relatively small and no formal assessment of patient comfort during the procedures was made. Notwithstanding this, we have documented in the same cohort a significant statistical difference between EUSFNA and ERCP-BB in the diagnosis of adenocarcinoma. The combined results were comparable to the best results of EUS-FNA with inroom cytology .We suggest that in the era of EUSFNA, biliary brushings at the time of ERCP should not be abandoned even when the procedure is performed simultaneously with EUS-FNA (particularly if in-room cytology is not available).
We also believe this study provides further evidence of the feasibility of combining EUS-FNA with ERCP and documents that this is possible under conscious sedation. Combining the procedures has potential benefits in shortening investigation time and lag to therapy [41].
Conflict of interest The authors have no potential conflict of interest