Keywords
|
| Pancreatitis, Acute Necrotizing; Tomography, X-Ray Computed |
Abbreviations
|
| EP: emphysematous pancreatitis |
INTRODUCTION
|
| Emphysematous pancreatitis (EP) is a rare and life-threatening necrotizing infection of the pancreas [1]. It is associated with gasforming bacteria and characterized by the presence of gas within or around the pancreas [2, 3, 4]. Computed tomography (CT) is the imaging modality of choice. It is both highly sensitive and specific in the detection of abnormal gas and is well-suited to reliably depict the anatomical location and extent of the gas. Although the identification of gas bubbles alone is not specific for the diagnosis of infection, their presence in an area of pancreatic necrosis on CT scan is considered a positive indicator of the presence of gasforming organisms [5]. Most reports on EP are based on radiological detection on CT scan [6]. Though the outcome of EP is reported to be poor, there are very few reports on the clinical characteristics of EP. We herein report our experience with EP and compare it with non-emphysematous infected pancreatic necrosis. |
PATIENTS AND METHODS
|
| The hospital records of all patients who underwent pancreatic necrosectomy for infected pancreatic necrosis between 2002 and 2006 were analyzed. In this time period, 172 patients with severe acute pancreatitis were seen. Fifty-seven underwent necrosectomy for infected pancreatic necrosis. In 11 out of these 57 (19.3%), gas was detected in the pancreatic bed on contrastenhanced CT scan. This subgroup of EP was reviewed in detail. Patients who developed gas after percutaneous intervention or surgery were excluded. |
| Patients with severe acute pancreatitis were managed using a standard protocol which included prophylactic antibiotics, nutrition (nasojejunal or parenteral) and supportive care for organ dysfunction. A CT scan was obtained for all patients. Image acquisition was at 5 mm intervals with intravenous contrast injected at the rate of 8 mL/second. The CT severity index (CTSI) [7] was calculated based on the extent of the necrosis and the number of fluid collections. Emphysematous pancreatitis was diagnosed when there was gas in the retroperitoneal, pancreatic bed on CT scan without any antecedent percutaneous or radiological intervention. Image-guided fine needle aspiration from pancreatic/peripancreatic tissue was done for microbiological studies if fever or leukocytosis persisted. Ultrasoundguided percutaneous drainage was employed as a temporary measure to drain localized peripancreatic collections. Contraindications to this procedure were the lack of an acoustic window and coagulopathy. A 9 French pigtailed catheter was placed using Seldinger’s technique. Patency was ensured by flushing with sterile normal saline. Review ultrasound was carried out every third day to confirm the position of the catheter and evaluate the size of the collection. The catheter was removed at the time of surgery when it acted as a guide to enter the lesser sac. Indications for surgical intervention were infected pancreatic necrosis, clinical deterioration, persistent organ dysfunction despite maximum intensive care and gas in and around the pancreas on CT. The necrotic material obtained at surgery was sent for aerobic and anaerobic bacterial cultures and the antibiotic therapy was changed according to the sensitivity report. All patients underwent pancreatic necrosectomy and post operative closed lesser sac lavage [8]. |
ETHICS
|
| All patients were treated according to usual clinical practice, following the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained before interventional procedures. There was no a priori approval by any institutional review committee because this was a retrospective analysis of hospital records. |
STATISTICS
|
| Patients with EP were compared to patients without EP in relation to age, etiology, CTSI, microbiological results, locoregional complications, organ failure, hospital course and mortality. Statistical analysis was performed using SPSS version 11. The Mann Whitney U test was used to compare continuous variables, and the Fisher’s exact and the chi-square tests were used for discrete variables. Two-tailed P values less than 0.05 were considered to be significant. |
RESULTS
|
| Fifty-seven patients with severe acute pancreatitis underwent necrosectomy for infected pancreatic necrosis between 2002 and 2006. Eleven of these patients (19.3%) had gas in and around the pancreas on CT scan. The clinical details of patients with EP and non-emphysematous infected pancreatic necrosis are compared in Table 1. There was no significant difference between the two groups with regards to age, gender and etiology. Patients in both groups presented predominantly in the mid-second to early third week of the onset of pancreatitis. |
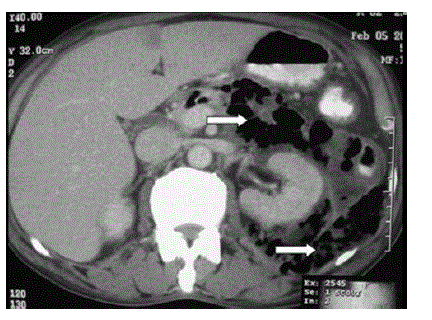
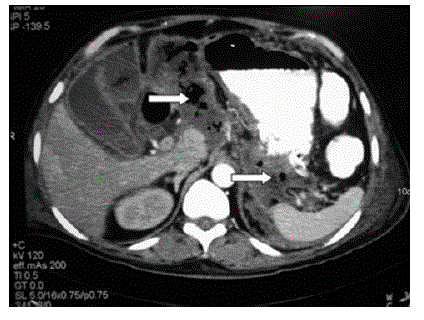
| The subgroup of patients with EP classically had necrosis of the pancreas with air specks, soft tissue stranding in the peripancreatic, perigastric, pararenal spaces and multiple intraabdominal fluid collections (Figures 1 and 2). The CTSI was 6 in one patient, 8 in four patients and 10 in six patients. The culture of the fine needle aspiration (FNA) or tissue obtained at necrosectomy grew Escherichia coli in all 11 patients. Pseudomonas aeruginosa and Acinetobacter aeromonas were the other commonly isolated microorganisms. The infection was polymicrobial in five patients and monomicrobial in six patients. The details of the bacteria isolated in the two groups are given in Table 2. |
| The timing of surgical intervention was in the fourth and fifth weeks of disease with no statistically significant difference between the two groups. Locoregional complications were encountered significantly more often in patients with EP (P=0.049). However, when the single complications were analyzed, there was no statistically significant difference in the incidence of gastrointestinal fistulae, bleeding, intra-abdominal collections or wound complications (P>0.250). Five out of the 11 EP patients (45.5%) developed multiorgan failure (defined as per Atlanta guidelines [9]). Respiratory failure was seen in all five patients, renal failure in three and hypotension requiring inotropes in two patients. The incidence of organ failure was similar in the two groups (P=0.297). Preoperative percutaneous drainage did not alter the outcome. One out of the five patients who underwent percutaneous intervention died in the EP group while 8 out of the 19 in the non-emphysematous infected necrosis group died (P=0.615). The median duration of the hospital stay and the mortality rate again did not differ significantly (P=0.580 and P=0.739, respectively) (Table 1). |
DISCUSSION
|
| EP is a rare variant of severe acute pancreatitis characterized by gas formation within and around the pancreas [1, 4, 10]. There are only scattered case reports in the literature, most of them dealing with radiological features. Attention was focused on EP after the advent of the CT scan. In a report of 450 patients undergoing CT for evaluation of a pancreatic pathology, 9 (2%) had intrapancreatic air, in 8 it was due to an abscess and in one due to an enteric fistula [11]. In another report of 259 patients, 7 (2.7%) had gas documented in the retroperitoneum [12]. In a recent report of 14 necrosectomies over 4 years, three patients had EP [13]. In the present series, 11 (19.3%) of the 57 patients undergoing necrosectomy for infected pancreatic necrosis had EP, all diagnosed on CT scan preoperatively. This is the largest clinical series on EP. |
| The details of management and outcome in EP have rarely been given in large series of patients undergoing necrosectomy. The word ‘emphysematous’ has serious connotations when prefixed to cholecystitis and pyelonephritis. EP has in fact been reported together with emphysematous infections of the gallbladder, kidney, urinary bladder and intestines [3]. This study compares the clinical course and outcome of EP with that of infected pancreatic necrosis which includes a seriously ill subgroup of severe acute pancreatitis with mortality rates as high as 40% [14]. |
| There is no known predisposition to EP. However, diabetes mellitus is known to predispose to gas gangrene [3]. There are also reports of EP associated with tuberculosis [15] occurring in HIV infected individuals [16]. In this study, the prevalence of diabetes was similar in patients with or without EP, and there were no specific factors contributing to immunocompromise [16]. |
| Most cases of EP have been attributed to gram negative organisms, the most common being Escherichia coli [11, 17], and others being Klebsiella species, Pseudomonas and Enterobacter [13]. Clostridium perfringes has also been implicated in gas gangrene of the pancreas [4, 17]. EP rarely occurs as a result of an infection with Mycobacterium tuberculosis [15]. In our study, all 11 patients with EP were infected with Escherichia coli, with 5 patients having polymicrobial infections. In contrast, Escherichia coli was found in 50% of the patients with infected pancreatic necrosis without EP (P=0.002) and was still the most common organism isolated. Polymicrobial infection was seen in 26.1% of the patients in the non-emphysematous subgroup. However, this difference did not reach statistical significance (Table 1). No anaerobic organisms were isolated in culture. |
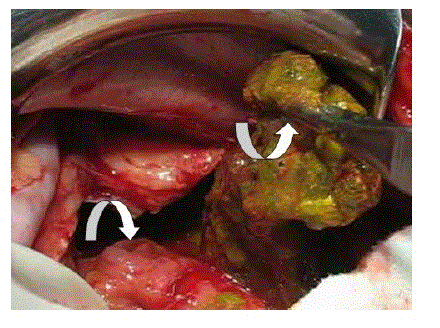
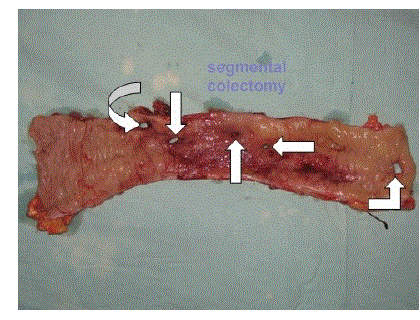
| The other important cause of EP is enteropancreatic fistula. The fistulization occurs between the pancreas and the duodenum, jejunum or colon. A suggestion has been made that the fistula may result from the rupture of a pancreatic pseudocyst [11]. One patient with EP had multiple fistulae between the pancreas, duodenum, jejunum and colon at the index operation. He required a left hemicolectomy, transverse colostomy and primary repair of the small bowel fistulae (Figures 3 and 4). Three other patients developed enteric fistulae in the postoperative period. One patient required a right hemicolectomy in a second operation; the other two were managed conservatively. |
| Interestingly, although the incidence of enteric fistulae was higher in patients with EP as compared to those with nonemphysematous infected pancreatic necrosis, (36.4% versus 19.6%), this did not reach statistical significance (Table 1). Endoscopic evaluation was not a routine preoperative investigation in patients with EP in this study and there was no way of predicting which patients with EP went on to develop enteric fistulae. Further studies are required to clarify this issue. |
| Gas within the pancreas is a rare occurence in patients with a patulous ampulla of Vater, duodenal diverticulum, penetrating duodenal ulcer or following instrumentation. Such patients may have a benign clinical course despite alarming radiological findings. In this study, EP refers to a subgroup of patients with infected pancreatic necrosis who invariably require intensive care. Image-guided percutaneous catheter drainage was employed as a temporizing measure prior to surgery in both groups (an average of 42% of patients) in order to drain localized collections. |
| Gas in the retroperitoneum was diagnosed in all 11 patients prior to any intervention. |
| It has been suggested that the presence of EP is an indication for surgical intervention [4, 10], although there is a recent report of successful conservative management of EP [1]. In this series, all 11 patients with EP underwent necrosectomy with closed lesser sac drainage and postoperative lavage, a technique which has been described elsewhere [8]. The recommended surgical approach is organ-preserving debridement combined with a postoperative management strategy which maximizes the evacuation of retroperitoneal debris and exudates [8]. The same approach was followed in patients without EP. |
| Contrary to the comments in the literature, patients with EP did not differ from those with non-emphysematous infected pancreatic necrosis in terms of severity of disease, duration of hospital stay and mortality. The incidence of locoregional complications and organ failure were also similar in the two groups. One possible explanation for this observation may be the strategy of intensive care offered to all patients with severe acute pancreatitis, and timely surgical intervention for them. Early and prompt recognition of EP is another aspect. |
| We conclude that gas in and around the pancreas on CT scan suggests a diagnosis of EP. This implies infected pancreatic necrosis which calls for intensive care and surgical intervention. With an aggressive management strategy, the clinical outcome of patients with EP can be expected to be similar to that in patients with non-emphysematous infected pancreatic necrosis. At the same time, we reiterate the need for prompt diagnosis of EP. |
Conflict of interest
|
| The authors have no potential conflicts of interest |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
References
- Ku YM, Kim HK, Cho YS, Chae HS. Medical management of emphysematous pancreatitis. J Gastroenterol Hepatol 2007; 22:455-6. [PMID 17295789]
- Birgisson H, Stef?nsson T, Andresd?ttir A, M?ller PH. Emphysematous pancreatitis. Eur J Surg 2001; 167:918-20. [PMID 11841083]
- Grayson DE, Abbott RM, Levy AD, Sherman PM. Emphysematous infections of the abdomen and pelvis: a pictorial review. Radiographics 2002; 22:543-61. [PMID 12006686]
- De Silva NM, Windsor JA. Clostridium perfringens infection of pancreatic necrosis: absolute indication for early surgical intervention. ANZ J Surg 2006; 76:757-9. [PMID 16916402]
- McCloskey M, Low VH. CT of pancreatic gas gangrene. Australas Radiol 1996; 40:75-6. [PMID 8838895]
- Daly JJ Jr, Alderman DF, Conway WF. General case of the day. Emphysematous pancreatitis. Radiographics 1995; 15:489-92. [PMID 7761653]
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331-6. [PMID 2296641]
- Wig JD, Mettu SR, Jindal R, Gupta R, Yadav TD. Closed lesser sac lavage in the management of pancreatic necrosis. J Gastroenterol Hepatol 2004; 19:1010-5. [PMID 15304118]
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993; 128:586-90. [PMID 8489394]
- Ghidirim G, Gagauz I, Misin I, Gutu E, Vozian M. Emphysematous necrotizing pancreatitis. Chirurgia (Bucur) 2005; 100:293-6. [PMID 16106939]
- Mendez G Jr, Isikoff MB. Significance of intrapancreatic gas demonstrated by CT: a review of nine cases. AJR Am J Roentgenol 1979; 132:59-62. [PMID 103405]
- Torres WE, Clements JL Jr, Sones PJ, Knopf DR. Gas in the pancreatic bed without abscess. AJR Am J Roentgenol 1981; 137:1131-3. [PMID 6976080]
- Lee VT, Chung AY, Chow PK, Thng CH, Low AS, Ooi LL, Wong WK. Infected pancreatic necrosis-- an evaluation of the timing and technique of necrosectomy in a Southeast Asian population. Ann Acad Med Singapore 2006; 35:523-30. [PMID 17006578]
- Mifkovic A, Pindak D, Daniel I, Pechan J. Septic complications of acute pancreatitis. Bratisl Lek Listy 2006; 107:296-313. [PMID 17125065]
- Morris DL, Wilkinson LS, al Mokhtar N. Case report: emphysematous tuberculous pancreatitis diagnosis by ultrasound and computed tomography. Clin Radiol 1993; 48:286-7. [PMID 8243012]
- Cho KC, Lucak SL, Delany HM, Morehouse HT, Jennings TA. CT appearance in tuberculous pancreatic abscess. J Comput Assist Tomogr 1990; 14:152-4. [PMID 2298985]
- Stockinger ZT, Corsetti RL. Pneumoperitoneum from gas gangrene of the pancreas: three unusual findings in a single case. J Gastrointest Surg 2004; 8:489-92. [PMID 15120375]
|