Yi Hu1, Zhi Su1*, Yangang He2, Yuling Liu2 and Fangfang Jiang1
1 School of Chemistry and Chemical Engineering, Xinjiang Normal University, Urumqi 830054, China
2 Institute of Microelectronics, Hebei University of Technology, Tianjin 300130, China
*Corresponding Author:
Zhi Su
School of Chemistry and Chemical Engineering
Xinjiang Normal University, Urumqi, China.
Tel: 00869914332416
E-mail: suzhixj@sina.com
Received Date: September 21, 2017; Accepted Date: September 27, 2017; Published Date: September 28, 2017
Citation: Hu Y, Su Z, He Y, Liu Y, Jiang F. Electrochemical Action of FA/O II Chelating Agent and H2O2 on Copper Film in the Polishing Process. Insights Anal Electrochem. 2017, 3:1. DOI: 10.21767/2470-9867.100021
Keywords
Electrochemical action; FA/O II chelating agent; H2O2; Copper film; Chemical mechanical polishing
Introduction
With the IC device feature size continues to decrease and the silicon wafer size continues to increase, the service life and the reliability of the device will to be improved. Then the wafer surface must achieve the global planarization. And chemical mechanical polishing (CMP) technology is now able to realize the uniqueness of the practical technology of wafer surface of global planarization. CMP is a complex technology that combines the mechanical friction of nanoparticles with the chemical reaction of the composition of the slurry to achieve the global planarization. Therefore, the chemical mechanical polishing liquid (slurry) should become the key factors that influence the quality of the products, which the performance determines the state of the material surface after CMP. And then, the follow-up process in the production process of IC will be affected. In order to assure the performance of the high-speed circuits, continuous efforts have been devoted to incorporating copper or low-dielectric constant (low-k) materials into multilevel interconnections to reduce the major part of circuit delay, cross talk, and power consumption. So, the trend of chemical mechanical polishing is that the pressure is getting lower and the polishing rate is increasing faster [1,2]. The kinds of polishing slurry general are mainly divided into acid and alkaline. The acidic polishing slurry is more popular; thus, the selective removal rate of different materials is poor. It is not conducive to the realization of global planarization. So, the slurry should be added BTA to form a passivation film on the surface to achieve the global planarization. But BTA is not conducive to the subsequent cleaning process. Therefore, it is imperative to develop the alkaline abrasive polishing slurry to suitable for low pressure conditions. In alkaline conditions, copper oxide and copper hydroxide is insoluble in water which is resulted in the alkaline route has not been realized [3,4].
But Hebei University of Technology successfully developed a kind of alkaline slurry which contained nano-SiO2 as abrasive, with FA/O II chelating agent, successfully achieved high speed and high surface quality in low pressure and low abrasive conditions.
This paper presented an investigation of the reaction mechanism of FA/O II chelating agents and H2O2 in the chemical mechanical polishing was studied by electrochemical test. The test methods were used by open circuit potential, polarization curve and cyclic voltammetry curve.
Materials and Methods
The slurry investigated was of colloidal silicate alkaline slurry (marked as HEBUT alkaline slurry), which included 10 wt% colloidal silica (the parameters are shown in Figure 2) in DI water liquid with a pH value of 10.5. The polishing solution contained the FA/O II chelating agent. The oxidizer (hydrogen peroxide) was added to the polishing solution in amounts ranging from 0 to 2 Vol%. The concentration of FA/O II chelating agent were from 0 to 5%. The electrochemical measurements were carried out by using LK2005B electrochemical workstation.
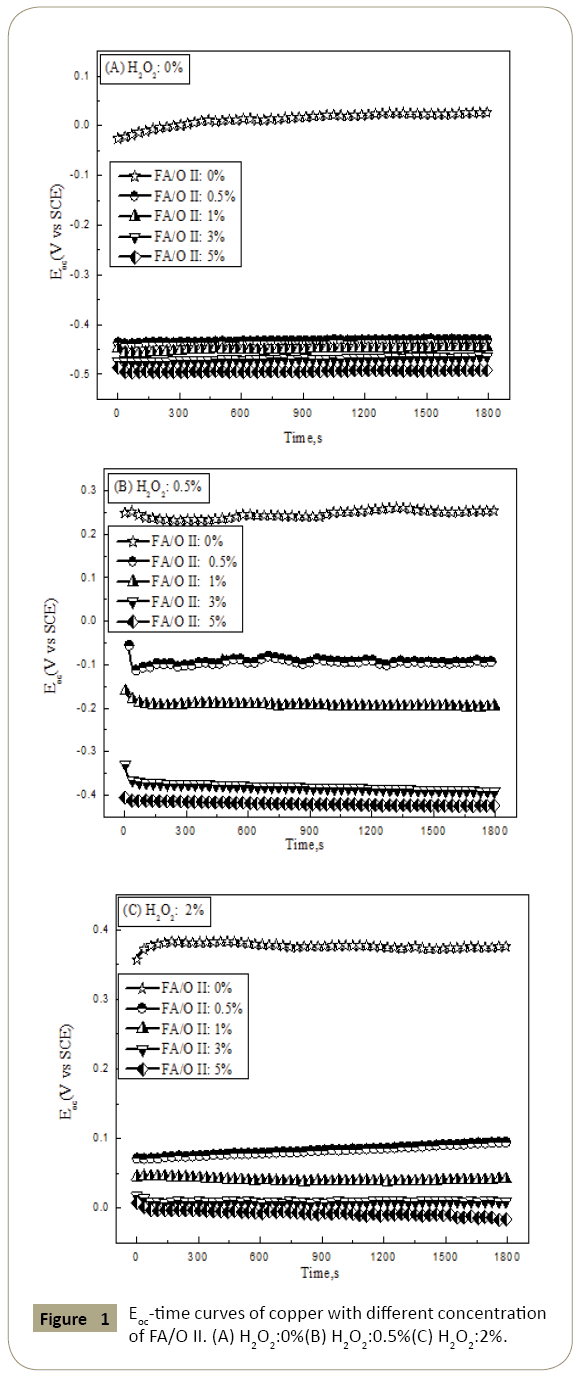
Figure 1: Eoc-time curves of copper with different concentration of FA/O II. (A) H2O2:0%(B) H2O2:0.5%(C) H2O2:2%.
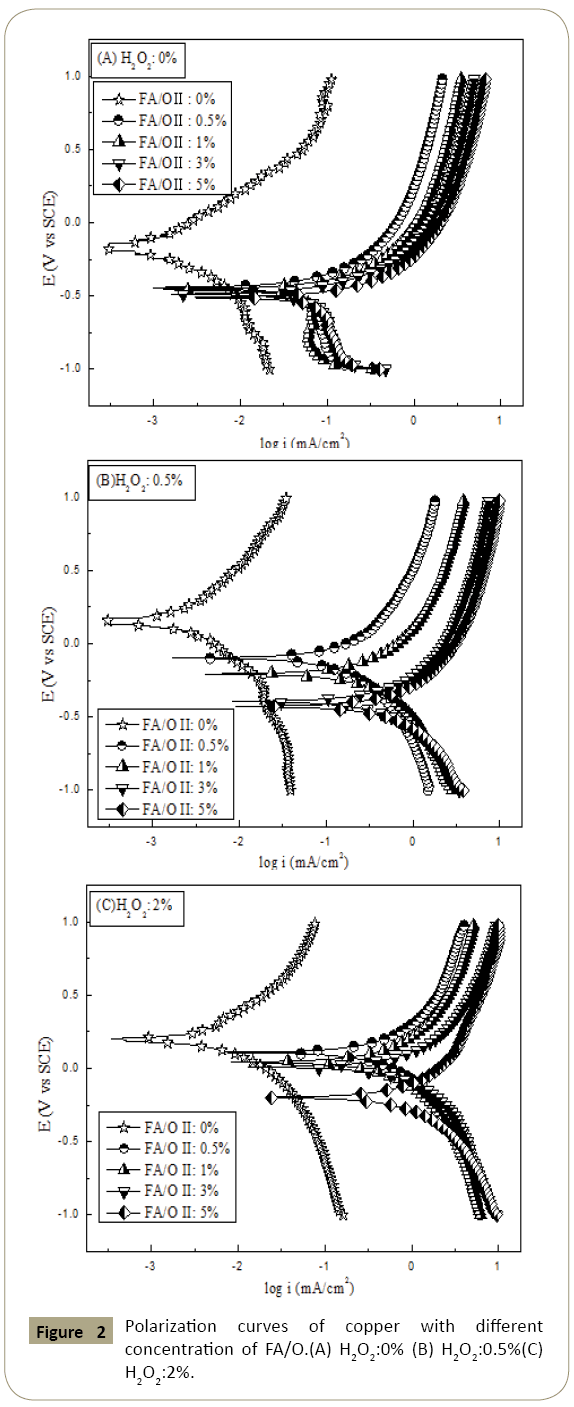
Figure 2: Polarization curves of copper with different concentration of FA/O.(A) H2O2:0% (B) H2O2:0.5%(C) H2O2:2%.
Results and Discussion
Electrochemical analysis of FA/O II chelating agent and H2O2
The reaction mechanism of chelating agents in chemical mechanical polishing was studied by electrochemical test. The test methods used include open circuit potential, polarization curve and cyclic voltammetry curve. The change curves of the open circuit potential of copper electrode in the polishing were showed in Figure 1. From the figures we could see that without adding the chelating agent and the concentration of H2O2 was 0%, the open circuit potential Eoc increased gradually with the time. This mainly because the dissolved oxygen in the solution made the copper surface gradually oxidized, and the oxides formed on the copper surface, which the electrochemical anodic reaction was hindered. Thus, the dissolved oxygen was limited, the open circuit potential Eoc was about 0.026 V in the whole process, but the electrochemical cathode reaction was enhanced after adding 0.5% H2O2, and copper was rapidly oxidized by H2O2. At the same time, the anodic reaction was inhibited. Therefore, in a short time after the copper electrode was immersed in the polishing slurry, the open circuit potential Eoc reached 0.25 V, and the open circuit potential was about 0.38 V after adding 2% H2O2.
After adding the chelating agent, the open circuit potential decreased significantly, and decreased with the increasing concentration of the chelating agent, which the main reason was that the chelating agent dissolved the oxide film, resulting in the fresh copper surface exposed to continue to participate in the electrochemical reaction thereby enhancing the electrochemical anodic reaction. In addition, it was observed that after 30 minute test, the open circuit potential tended to be stable, indicating that the whole system reached steady state [5,6].
Analysis of polarization curves of copper
The polarization curves of copper in different solutions were showed in Figure 2. As the polarization curves of the three diagrams were not coincident, the curves could be analyzed directly from the location of the curve and the corresponding self-corrosion potential. It could be seen that when the concentration of chelating agent was 0%, the copper surface showed a passivation state and the corrosion current was at a low value. After adding the chelating agent, with the increase of the concentration of chelating agent, polarization curve moved right and corrosion potential decreased, which showed that the solution of electrochemical reaction was more intense and the complex interaction was enhanced. The corrosion probability increased greatly, which improved the corrosion rate of copper. At the same time, after adding the chelating agent the polarization curve did not show the self-passivation phenomenon obviously, probably because of the chelating agent dissolved the oxide film. The copper oxide film surface became porous, which contributed to the new copper surface to continue to participate in the electrochemical reaction. So, the polarization curve would not show the passivation state. Figure 2 showed that when there was no H2O2 in the solution, the cathode reaction was the main reaction, and oxygen diffusion controlled. When H2O2 was added, the electrochemical cathode reaction was enhanced. Now, the oxidation of H2O2 was obviously increased, and the thickness of the passivation layer increased rapidly, so the oxygen diffusion control did not appear before.
Analysis of open circuit potential Eoc and corrosion potential Ecorr
The polarization curves parameters were as shown in Figures 3 and 4. In figures the open circuit potential Eoc and corrosion potential Ecorr changed with the concentration of chelating agent which confirmed the analysis on polarization curve above. With the increase of the concentration of chelating agent, the open circuit potential and the corrosion potential decreased, which meant the corrosion probability increased. Thus the increased corrosion probability implied that the passivation film would be rapidly decomposed by the chelating agent. The protective effect of passivation layer on the copper surface disappeared, and the exposed surface of the fresh copper continued to participate in the electrochemical reaction. Thereby it would enhance the electrochemical anodic reaction of the whole circuit. It was observed that when the concentration of chelating agent was greater than 1% and the H2O2 was 0% and 2% respectively, the open circuit potential increased indistinctly with the concentration of chelating agent, while the corrosion potential decreased continuously when H2O2 was 0.5%. Without H2O2, oxygen diffusion was mainly controlled, and electrochemical anodic reaction and cathode reaction were inhibited, which resulted in no change of open circuit potential.
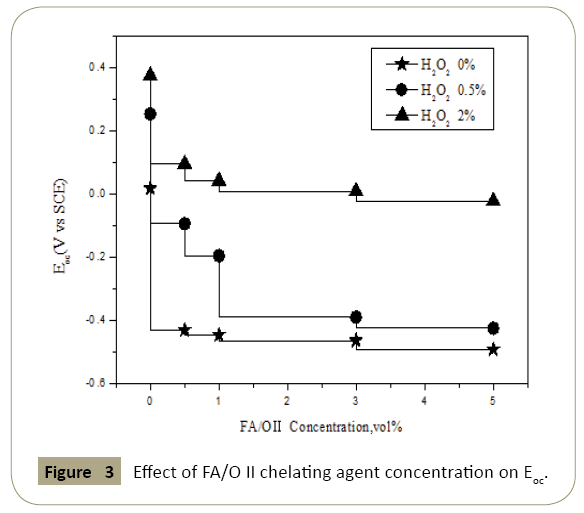
Figure 3: Effect of FA/O II chelating agent concentration on Eoc.
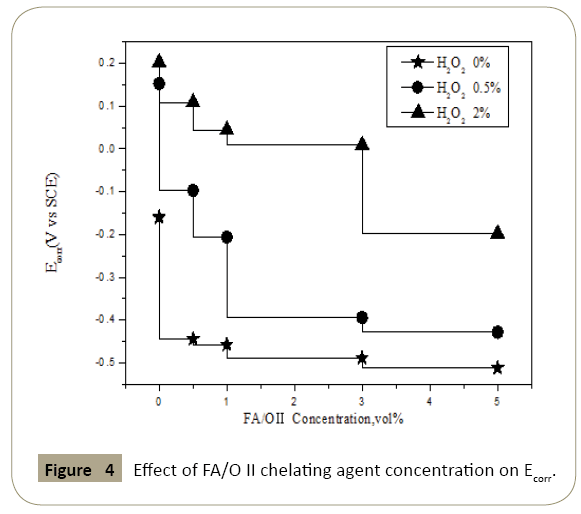
Figure 4: Effect of FA/O II chelating agent concentration on Ecorr.
When the concentration of H2O2 was 2%, the oxide film produced on the copper surface was thicker and denser, and the chelating agent could not effectively eliminate the sufficient passivation layer, which led to a great inhibition of electrochemical reaction. In addition, it could be seen from the chart, although the change trend of open circuit potential and corrosion potential was approximately the same, the corresponding values were not the same, the reason was probably that in the polarization curve measurement process, the applied potential to the copper electrode could activate the multiple reactions in the potential system. These reactions changed the chemical composition of the copper surface, and thus the equilibrium potential varies with the system without the applied voltage.
Cyclic voltammetry curves of copper
The cyclic voltammetry curves of copper in different solutions corresponding to the peak current of the anode were shown in Figure 5. As could be seen from the figure, after adding H2O2, the cathode peak current and peak anode current was greater than no H2O2 content. The addition of H2O2 enhanced the oxidation reaction. And it also enhanced the chelate reaction between chelating agent and copper ions, accelerated the dissolution of copper, which also enhanced the electrochemical anodic reaction. From the figure we also could see that there was no obvious oxidation-reduction current peak in the cyclic voltammetry curve and no passivation zone appeared.
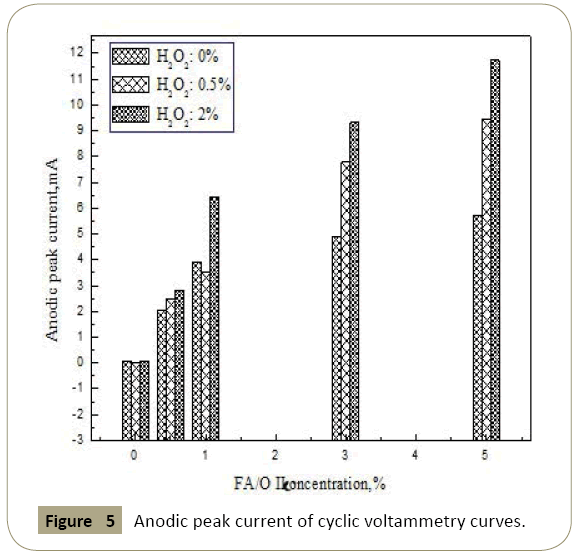
Figure 5: Anodic peak current of cyclic voltammetry curves.
When there was H2O2, with the increase of the concentration of chelating agent, the anodic peak current increased. This showed increasing the concentration of chelating agent would help oxide film dissolved, and made the oxide film on copper surface thin and porous. So fresh copper surface exposed to continue to participate in the electrochemical reaction, and enhanced electrochemical anodic reaction to the entire loop. This was consistent with the test results obtained by using open circuit potential and polarization curve techniques.
Conclusion
Previous experiments proved that H2O2 obviously had an effect on the removal rate of copper film under low pressure and low abrasive CMP condition. The polishing rate increased with the increase of H2O2 content, and reached the maximum value when the content of H2O2 exceeded a certain concentration, the removal rate decreased. This was because the addition of H2O2 in polishing slurry, which would release the strong oxidation of OH+. The surface of the copper could be rapidly oxidized which promoted the formation of copper oxide film and the oxide film thickening density. And the corrosion potential of the open circuit potential was increased. Based on the above analysis of the electrochemical principle in the low abrasive CMP process, the copper surface area was removal and chelating agent reaction complexation was to form soluble products. The products were taken away pass through the abrasive and polishing pad. After polishing, the fresh copper surface was exposed, and chemical reactions with the polishing slurry continued to produce the new oxide film. When the concentration of FA/O II chelating agent was 0.5% and the concentration of H2O2 was 0.5%, the excessive corrosion could be avoided, and the selectivity of removal rate of copper surface would be improved, and the planarization efficiency could be improved.
This process goes around and round. While the copper oxide film by preventing excessive protection, polishing rate is very slow. It could improve the polishing selectivity of copper polishing process and effectively improve the planarization efficiency.
Acknowledgments
Funded by Scientific Research Program of the Higher Education Institution of XinJiang (XJEDU2016S064); Doctoral Program Foundation of Xinjiang Normal University Plan(XJNUBS1226); College students innovative entrepreneurial training project (201710762098).
References
- Li Y,Sun M,Niu XH, Liu YL, He YG, et al. (2014) Removal of residual CuO particles on the post CMP wafer surface of multi-layered copper. Journal of Semiconductors 35:1-5.
- Rodriguez JB, Madiomanana K, Cerutti L (2016) X-ray diffraction study of GaSb grown by molecular beam epitaxy on silicon substrates. J Cryst Growth 439: 33-39.
- Li Y, Liu YL, Wang AC, Yang ZX, Sun MB, et al. (2014) Research on the application of Cu/SiO2 catalytic system in chemical mechanical planarization based on the stability of SiO2 sol. Journal of Semiconductors 35:1-7.
- Chen H,He YY,Lin MH (2016) Characterizations of zinc oxide nanorods incorporating a graphene layer as antibacterial nanocomposite on silicon substrates. Ceram Int 42: 3424-3428.
- Abd MN, Rahman R, Yusuf Y,Mansor M (2016) Effect of nitridation surface treatment on silicon (111) substrate for the growth of high quality single-crystalline GaNheteroepitaxy layer by MOCVD. Appl Surf Sci 362: 572-576.
- Megouda N (2016) Investigation of morphology, reflectance and photocatalytic activityof nanostructured silicon surfaces. Microelectron Eng 159: 94-101.