- (2008) Volume 9, Issue 2
Brian M Yan1, Reetesh K Pai2, Jacques Van Dam1
1Division of Gastroenterology and Hepatology, and 2Department of Pathology; Stanford University Medical Center. Stanford, CA, USA
Received December 10th, 2007 - Accepted January 18th, 2008
Context Gastrointestinal stromal tumors of the pancreas are very rare. Only two case reports have been published, both with diagnoses made on surgical pathology. We present the first case of pancreatic stromal tumor diagnosed by endoscopic ultrasound guided fine needle aspiration. Case report A 47-year-old male presented with self limited nausea and vomiting. A CT scan revealed a subtle, hypervascular mass in the uncinate process of the pancreas. Endoscopic ultrasound confirmed the pancreatic mass and fine needle aspiration was performed giving a bloody sample. Cytology showed spindle cell proliferation with CD117 positive immunohistochemistry, confirming a pancreatic gastrointestinal stromal tumor. Conclusion We present a case of pancreatic stromal tumor diagnosed by endoscopic ultrasound guided fine needle aspiration. Although very rare in the pancreas, gastrointestinal stromal tumors should be considered in the differential diagnosis of solid pancreatic masses and blood aspirates. INTRODUCTION Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumor of the gastrointestinal GI tract and occur primarily in the stomach (40-50%) and small bowel (30-40%) [1, 2]. Primary GISTs of the pancreas are extremely rare and previously have only been diagnosed by surgical pathology [3, 4]. We describe the first case of pancreatic GIST diagnosed by EUS-guided FNA.
Biopsy, Fine-Needle; Endosonography; Gastrointestinal Stromal Tumors; Pancreas
GI: gastrointestinal; GIST: gastrointestinal stromal tumor
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumor of the gastrointestinal GI tract and occur primarily in the stomach (40-50%) and small bowel (30-40%) [1, 2].
Primary GISTs of the pancreas are extremely rare and previously have only been diagnosed by surgical pathology [3, 4]. We describe the first case of pancreatic GIST diagnosed by EUS-guided FNA.
A 47-year-old male experienced a self-limited episode of nausea and bilious vomiting with no other significant gastrointestinal symptoms or constitutional features. Past medical history included chronic hepatitis B with liver cirrhosis, esophageal varices, and prior removal of a benign scrotal mass. The patient’s medications included lamivudine, adefovir, pantoprazole, and propanolol. Physical examination revealed splenomegaly, but otherwise was unremarkable.
Laboratory investigations were noncontributory. Upper endoscopy revealed esophageal varices, portal hypertensive gastropathy, and a nodular duodenal bulb (negative on biopsy.) An abdominal CT scan showed a 2.4x2.1cm hypervascular pancreatic mass (Figure 1). Radial and linear EUS with Doppler examination (Olympus GFUM-160 and GFUC-140P, Olympus America, Melville, NY, USA) revealed a round hypoechoic mass in the uncinate process with small peripheral vessels (Figure 2). No vascular flow was evident within the mass or in the needle path. EUS-FNA with a 19-gauge needle (Echotip, Cook Endoscopy, Winston- Salem, NC, USA) demonstrated a soft mass, with no blood aspirated initially. However, after repeated movements of the needle, the aspirate became frankly bloody. Two passes were made without complication.
Cytology on cell block preparation revealed spindle cell proliferation with no significant nuclear enlargement, mitotic activity or necrosis. Immunohistochemistry showed diffuse, strongly -positive staining for CD117 and negative for desmin, all supporting a diagnosis of GIST (Figure 3).
Most pancreatic masses are adenocarcinoma, however practitioners should be aware of other possibilities which have different (and usually better) treatment and prognoses. Pancreatic GISTs are very rare with two cases reported in the literature [3, 4]. Both patients were diagnosed with high-risk GISTs based on pathology of surgically-resected specimens. To the authors’ knowledge, this is the first reported case of primary low-risk pancreatic GIST diagnosed by EUS-FNA. Successful diagnosis of luminal GIST via FNA is widely variable in the literature ranging from 38% to 89%, partially depending on the availability of an on site cytopathologist to determine adequacy of a sample [5, 6, 7, 8]. There is still debate on the utility of FNA, or the use of Trucut biopsy, for the diagnosis of GIST. FNA on solid pancreatic lesions has a higher and more consistent accuracy rate of 75-96%, however adenocarcinoma appears to have a higher accuracy rate compared to other solid lesions [9, 10, 11]. In this case, FNA with two passes of a 19-gauge needle provided sufficient material for cytologic and immunohistochemical analyses.
The cytologic differential diagnosis for spindle cell proliferation includes leiomyoma, schwannoma, GIST, fibromatosis, inflammatory fibroid polyps, and gastrointestinal muscularis sampling. Immunohistochemistry demonstrating CD117 positivity confirms the diagnosis of GIST. Cases of fibromatosis have been reported in the literature to react with antibodies directed against CD117, although this does not typically involve the pancreas [12]. GIST primarily occurs within the luminal GI tract, but practitioners should be aware that they can arise from outside the GI tract. GISTs are thought to originate from interstitial cells of Cajal of the GI tract. If they can arise from other organs, then one may consider a subset of GISTs arising from other cells or the presence of cells of Cajal within that organ. The two prior cases of pancreatic GIST were diagnosed on surgical specimens indicating that GISTs can arise from the pancreas.
An alternative possibility for the diagnosis of a pancreatic GIST is one originating from the duodenum immediately adjacent to the pancreas. The proximity of the C-loop of the duodenum to the pancreatic head may make it difficult to distinguish the origin of the lesion on cross sectional imaging alone. A recent report has highlighted this possibility; however, EUS was not used in the preoperative workup of the GIST lesion [13]. Given its ability to distinguish the layers of the GI tract with high accuracy, EUS is unequivocally the best modality to determine if the lesion is arising from the wall of the duodenum. This was not the case in our patient.
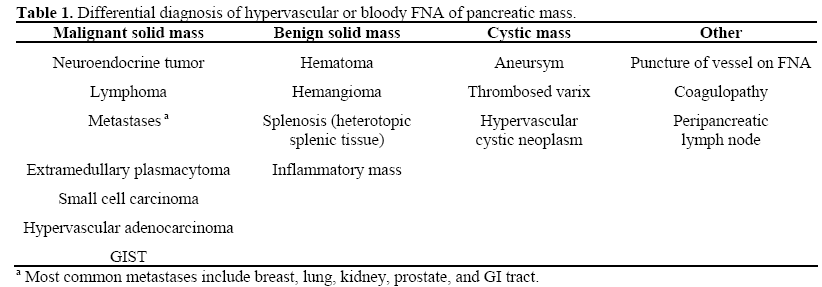
While literature on the etiology of a franklybloody FNA of the pancreas is lacking, the differential diagnosis for hypervascular pancreatic lesions can theoretically be applied to bloody aspirates (Table 1). The most common cause is trauma to a small vessel in the needle path not seen on EUS/Doppler, while neuroendocrine tumors are the most likely primary lesion. A recent report by Varadarajulu and Eloubeidi demonstrated a 0.97% rate of aneurysms masquerading as pancreatic cystic neoplasm [14]. Given this risk, the authors suggested against further EUS-FNA attempts if clear blood is aspirated. However, in our experience and that of many others, a bloody aspirate is not necessarily a contraindication for repeated FNA. Multiple passes are often required to attain a sufficient aspirate for analysis. As demonstrated in this case, a hypervascular lesion can result in frank blood in the aspirate. The diagnosis may have been missed if the FNA was inadequate due to not enough needle passes.
In summary, we present a rare case of pancreatic stromal tumor diagnosed by EUSguided FNA. Although rare in the pancreas, gastrointestinal stromal tumors should be considered in the differential diagnosis of solid pancreatic masses.