- (2006) Volume 7, Issue 1
Ralf Wilkowski1, Martin Thoma1, Christiane Bruns2, Eckhard Dühmke1, Volker Heinemann3
1Clinic for Radiation Oncology, 2Surgical Clinic, and 3Medical Clinic III, LMU University Hospital Grosshadern. Munich, Germany
Received: 14 October 2005 Accepted: 3 November 2005
Context Primary resectability is expected in up to 20% of pancreatic cancer patients. While most patients relapse with distant metastases, approximately 30% of patients show isolated local recurrence without evidence of distant metastases. Objective The present analysis investigates the efficacy of chemoradiotherapy in this particular patient group.. Design Retrospective study. Patients Eighteen consecutive pancreatic cancer patients presenting with isolated locoregional recurrence after surgical resection. The median interval between primary surgery and diagnosis of local recurrence was 10.4 months (range: 2.0-19.3 months). Interventions Patients received 3-D conformal radiation with 45 Gy in 25 fractions of 1.8 Gy/day. Simultaneous chemotherapy was employed either with continuous 5-FU infusion, partly in combination with gemcitabine, or with gemcitabine and cisplatin. Sequential chemotherapy with gemcitabine and cisplatin was given to some patients before and after the chemoradiotherapy. Results In 17 of the 18 patients included, radiotherapy was employed at the intended dose. While WHO grade 3-4 gastrointestinal toxicity was not reported, hematotoxicity was more pronounced. Grades 3 and 4 leukocytopenia occurred in 4 patients (22.2%) and 1 (5.6%) patient, respectively, and grades 3 and 4 thrombocytopenia occurred in 4 patients (22.2%) and 1 patient(5.6%), respectively. Six (37.5%) complete remissions, 6 (37.5%) partial remissions, and 4 (25.0%) stable diseases were noted in 16 evaluable patients. Median progression-free survival calculated from the start of the chemoradiotherapy was 14.7 months (range: 8.4-21.0 months) . Seven (28.9%) patients had another local relapse, while 11 (61.1%) patients developed distant metastases. Median overall survival from the start of the chemoradiotherapy was 17.5 months (95% CI: 15.6-19.4 months) and median survival from the initial diagnosis was 27.2 months (95% CI: 23.9-30.6 months). Conclusion The data provide a first indication that chemoradiotherapy is feasible and may be an effective treatment option in those patients who present with local metastasis after primary surgery for pancreatic cancer.
Combined Modality Therapy; Neoplasm Recurrence, Local; Pancreatic Neoplasms /surgery
AJCC: American Joint Committee on Cancer; CRT: chemoradiotherapy; CTC: common toxicity criteria; GITSG: Gastrointestinal Tumor Study Group; ICRU: International Commission on Radiation Units and Measurements; ECOG: Eastern Cooperative Oncology Group; UICC: International Union Against Cancer
Despite intensive interdisciplinary efforts, the prognosis for patients with a pancreatic carcinoma continues to be dismal. Approximately 20% of patients are curatively resectable at first diagnosis [1] which is mainly due to the absence of early diseaserelated symptoms. After a complete resection of the primary tumor, the risk of loco-regional relapse is about 35-86% in pancreatic cancer [2, 3, 4, 5]. Despite the use of a more radical surgical approach with extensive lymph node dissection, substantial improvement has not been achieved [6].
In the majority of patients, tumor progression occurs not only locoregionally, but predominantly also as a distant metastasis, specifically in the liver, peritoneum or lungs. There is, however, a smaller group of patients (approximately 30%), where isolated local recurrence is observed without evidence of distant metastasis [4, 7, 8]. Following extended surgical pretreatment, there is, for the most part, no further surgical option in local relapse. Therefore, it may be assumed that radiotherapy has the greatest impact on locoregional disease control.
At present, gemcitabine is considered the agent of choice for treatment of advanced or metastatic pancreatic cancer inducing an improved survival time when compared to 5- FU alone [9, 10]. With gemcitabine-based combination therapies, especially cisplatin and 5-FU, an additional improvement of tumor response and time to progression has been reported [11, 12, 13]. Several trials using simultaneous chemoradiotherapy (CRT) with gemcitabine, 5-FU or cisplatin have shown that high efficacy can be achieved locally [14, 15, 16].
Based on trials performed by the Gastrointestinal Tumor Study Group (GITSG), combined CRT is suggested to be superior to chemotherapy or radiotherapy alone in patients presenting with de novo locally advanced pancreatic cancer [17, 18], although, so far, no information is available regarding the importance of CRT applied in local recurrence of pancreas cancer after surgical pretreatment. For this reason, a retrospective analysis was carried out in this subgroup of patients to investigate the efficacy of 5-FU- or gemcitabine-based CRT with regard to local efficacy, survival and toxicity.
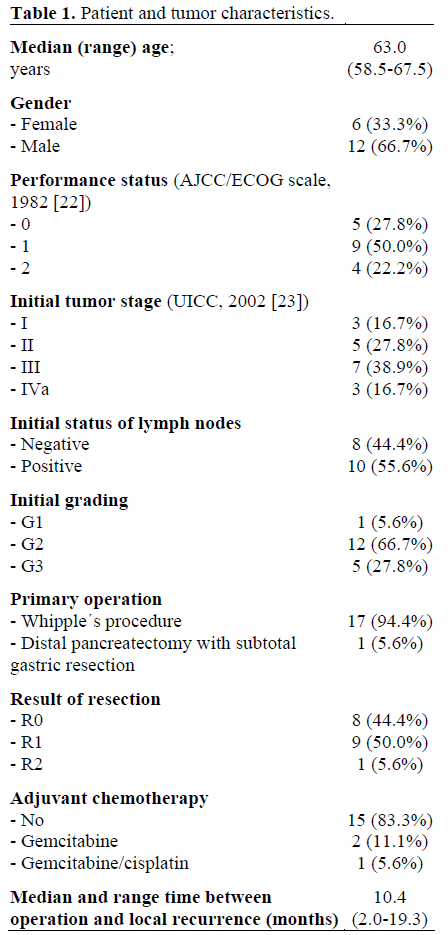
Patients
Between August 1997 and March 2004, eighteen patients with isolated local recurrence after surgical resection of pancreatic cancer were treated at a single institution. There were 6 women and 12 men; the median age was 63 years. The median interval between surgery and the diagnosis of local recurrence was 10.4 months (range: 2.0- 19.3 months). Fifteen patients (83.3%) had not received postoperative treatment; the other three patients had undergone postoperative adjuvant chemotherapy, two with gemcitabine (11.1%), and the third with gemcitabine and cisplatin (5.6%). Relevant patient and tumor characteristics as well as pretreatment data are summarized in Table 1. The diagnosis of local recurrence was established by CT- or MRI-imaging, which was requested based on increased CA 19-9 levels in 9 of the 18 patients. Patients were included in the trial if they had a Karnofsky index equal to or greater than 70%, and adequate liver and kidney function. Systemic metastasis was excluded by means of thoracic and abdominal CT-imaging. Moreover, prior radiation was defined as an exclusion criterion whereas other adjuvant treatment such as chemotherapy was permitted. At primary diagnosis, all patients had undergone tumor resection with curative intention.
Patients were treated prospectively with existing analog protocols for primarily unresectable pancreatic cancer. Since isolated local recurrent pancreatic cancer is rarely treated, in the specified period, not enough patients could be treated in a proprietary protocol. Data collection was primarily by chart review. The patients were identified the registry databases of our institutions.
A three-dimensional exposure plan (HELAXTM) was developed for all patients. In 13 patients, the macroscopically visible tumor as well as the loco-regional lymph nodes were irradiated with an ICRU (International Commission on Radiation Units and Measurements) reference dose of up to 45.0 Gy applied in 25 fractions with 1.8 Gy/day. In five patients, the macroscopic tumor was given a dose of up to 50.0 Gy in the ICRU reference, wherein the field arrangement was calculated so that the loco-regional lymph drain was included as a second order target volume by a 90% isodose and hence given a 45.0 Gy dose.
The dose given to the adjoining organs at risk was calculated by means of dose volume histograms. Less than 40.0 Gy were applied to the spinal cord; the liver received a maximum of 25.0 Gy in 50% or 37.5 Gy in 25% of the organ. The kidneys were exposed to a maximum of 20.0 Gy in 50% (right) and 30% (left) of the organ.
Ten patients were given daily simultaneous chemotherapy treatments with 5-FU 350 mg/m2 as a continuous infusion. Six of these patients additionally received gemcitabine 300 mg/m2 as a 30-minute infusion on days 1, 15, and 29.
While receiving irradiation, eight patients were given cisplatin 30 mg/m2 over one hour and gemcitabine 300 mg/m2 over 30 minutes on treatment days 1, 8, 22, and 29.
Sequential chemotherapy with gemcitabine 1,000 mg/m2 and cisplatin 50 mg/m2 was given to six patients before and 11 patients after CRT on days 1 and 15 (1 cycle before and 2 cycles after CRT).
Toxicities related to CRT were assessed and documented according to the common toxicity criteria (CTC) for the grading of the acute and subacute side effects [19].
Response to the therapy was recorded on completion of the simultaneous CRT and in 8- 12 week intervals thereafter. Staging analyses included the testing of blood counts, the analysis of serum chemistry and tumor markers CEA und CA 19-9, as well as CTscans of the thorax and abdomen. A complete response required no evidence of tumor on post-treatment CT scan. A partial response required a 50% reduction of the maximum perpendicular tumor measurements.
Informed consent was obtained from each patient. Treatment was performed conforming to the ethical guidelines of the "World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, as revised in Tokyo 2004.
The target parameters of overall survival, survival from the beginning of recurrencetreatment and progression-free survival were estimated using the Kaplan-Meier method. Treatment-associated toxicity was evaluated according to CTC-criteria [19]. Data were analyzed by using the SPSS for Windows Version 11.5.1.
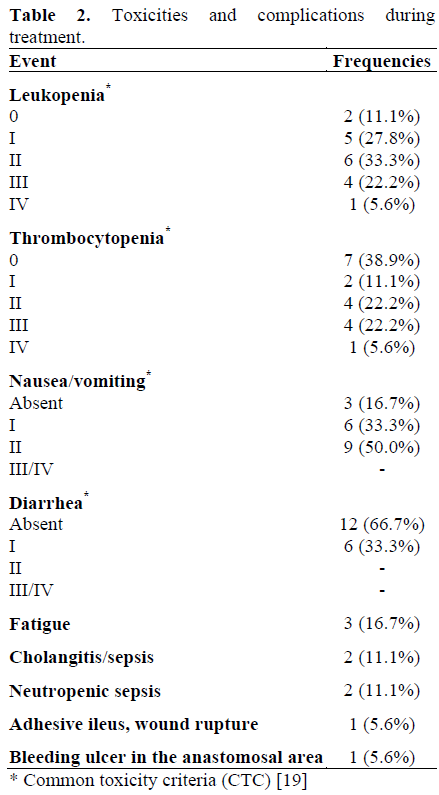
In 17 of the 18 patients included, it was possible to give radiotherapy at the intended dose, whereas the remaining patient was diagnosed with upper bowel obstruction due to extensive postoperative adhesions, which required surgical intervention. In this latter patient, the radiotherapy was interrupted for 14 days; then, after radiotherapy was begun again, the patient developed wound healing problems in the upper abdomen, and further surgery was performed. After application of 41.4 Gy, radiotherapy was stopped.
Simultaneous chemotherapy was given to all patients at full dose. Sequential chemotherapy, by contrast, had to be reduced to 50% of the intended dose in two patients due to leukopenia. One of these patients refused further chemotherapy after the first application; in the second patient, treatment was terminated after the third application of chemotherapy due to neutropenic sepsis.
With the administration of adequate supportive care including ondansetrone and dexamethasone, no grade 3 or 4 gastrointestinal toxicities occurred during concurrent chemoradiation, as indicated in Table 2. As expected, hematotoxicity was more pronounced. Grade 3 leukopenia occurred in four patients (22.2%; two receiving simultaneous gemcitabine/cisplatin, two receiving gemcitabine/5-FU). Grade 3 thrombocytopenia was documented in four patients (22.2%; two patients with simultaneous gemcitabine/cisplatin, one with 5-FU, and one with gemcitabine/5-FU). One patient (5.6%) developed grade 4 leukocytopenia and thrombocytopenia, after which chemotherapy was discontinued. A further patient developed a bleeding ulcer of the gastrojejunal anastomosis. Relevant late complications did not occur in association with the treatment.
Treatment Efficacy
At the time the data were recorded in April 2005, two of the 18 patients treated were still alive (11.3 and 27.3 months after diagnosis of recurrence) without distant metastasis.
Sixteen patients were evaluable for response analysis. Six patients (37.5%) achieved complete remission and another six patients (37.5%) partial remission. Stable disease was achieved in four patients (25.0%) (Table 3). No patient developed localized tumor progression during the CRT.
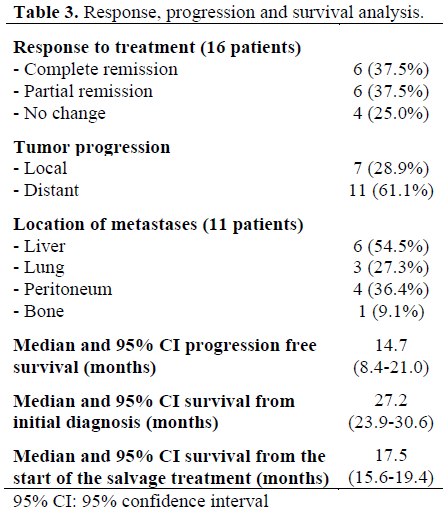
Median progression-free survival after the start of salvage treatment was 14.7 months (95% CI: 8.4-21.0 months). Local tumor progression occurred in seven patients (median 14.7 months after the start of salvage treatment) and 11 patients developed distant metastases (median 11.0 months).
One patient was lost to follow-up 5.6 months after CRT. Median overall survival after the start of salvage treatment (including the dropout patient) was 17.5 months (95% CI: 15.6-19.4 months). Median overall survival after the initial diagnosis of pancreas cancer was 27.2 months (95% CI: 23.9-30.6 months).
Improvement of pancreatic cancer outcome continues to be a major interdisciplinary challenge. Even after initial tumor resection is conducted with curative intention, locoregional recurrence is reported in up to 86% of patients. [2, 3, 4, 5].
Pancreatic cancer patients who, after curative surgery of the primary tumor, experience local recurrence without distant metastasis, appear to be a subpopulation with a more favorable prognosis. While several studies support this notion [7, 8, 20], no data are so far available on the efficacy of CRT in these patients.
Therefore, this is the first evaluation of CRT applied to patients with isolated local metastases after primary surgery. The median survival of 17.5 months from the start of the salvage treatment described here supports the efficacy of this approach. Patients with locally advanced unresectable pancreatic cancer, who are initially treated with CRT, achieve median survival times ranging from 10.3 to 13.3 months in various studies [16, 21]. Hence, it appears that patients with isolated local recurrence after primary surgery have a median survival comparable to those patients with initially unresectable disease, when both groups receive CRT.
Compared to other trials, we noticed a very low rate of gastrointestinal toxicities. Although this could be due to the retrospective collection of toxicity-data and the limited number of patients, we feel that our intensive supportive care and inpatient treatment and observation is responsible for these results.
Clearly, this analysis is limited by its retrospective nature and by the low number of patients included. Nevertheless, the data provide a first indication that CRT is feasible and may be an effective treatment option in those patients who present with local failure after primary surgery of pancreatic cancer.