- (2015) Volume 16, Issue 6
Mallikarjuna Uppara1, Patooru Vijaya kumar2, Singaram Palaniappan3, Ravi Ramakrishnan4, Rajagopal Surendran5, Ashraf Rasheed6
1University Hospital of Wales, Cardiff CF14 4XW
2General Surgery, Apollo Hospitals, Chennai, India
3Gastroenterology, Global Hospitals, Chennai, India
4Gastroenterology, Fortis Hospital, Chennai, India
5Surgical Gastroenterology, Apollo Hospitals, Chennai, India
6Department of Upper GI Surgery, Royal Gwent Hospital, Newport, Wales, United Kingdom
Received May 20th, 2015-Accepted July 20th, 2015
Objective To retrospectively study the correlation between pre-operative morphological and biochemical features of resected pancreatic cystic lesions and predictive power of these features in relation to biological behaviour and final histology. Methods We reviewed the literature systematically to identify relevant variables that are in use to predict the biological nature of pancreatic cystic lesions and aid therapeutic planning. We designed a template encompassing all used variables to collate the available data of resected pancreatic cystic lesions from two centres. The collated data included clinico-pathological and biochemical data, pre-operative computed tomography, magnetic resonance imaging, Endoscopic ultrasound, positron emission tomography–computed tomography, Fine-needle aspiration analysis whenever available and correlated with the final post-operative histology. Pooled data was analysed using statistics and data 14 statistical software. Results Sixty-four patients with pre-operative diagnosis of pancreatic cystic lesions were identified. Twenty seven cases underwent endoscopic ultra sound - fine-needle aspiration as an adjunct to the radiological assessment to evaluate the nature of these noted PCLs and both cytological and biochemical analysis were carried out on the intra-cystic aspirate. The intra cystic carcinoembryonic antigen levels recorded a mean of 667.97 in the tested group with a standard deviation of 1934.38. Conclusion No single test is able to predict the nature or behaviour of pancreatic cystic lesions. The differences noted on specialist imaging can be very subtle and demand specialist interpretive skills and hence a panel of pre-operative testing with review at specialist multidisciplinary meeting is mandatory for all such cases.
Pancreas; Pancreatic Cysts
CT computed tomography; CEA carcinoembryonic antigen; DAC: Ductal adenocarcinoma; EUSCFA endoscopic ultra sound guided cyst fluid aspiration; FNA fine needle aspiration; IPMN intraductal papillary mucinous neoplasm; MCN mucinous cystic neoplasm; MPD main pancreatic duct; MRI magnetic resonance imaging; PC psuedocyst; ScyA serous cyst adenoma; STATA statistics and data; VHL Von Hippel-Lindau Syndrome
Cystic lesions of pancreas may be asymptomatic or present with epigastric pain, nausea, steatorrhea, abdominal discomfort, weight loss, vomiting, jaundice, backache or diarrhoea.
Pancreatic resection continues to carry high morbidity (69%) for total pancreatectomy [1] and (20%) risk of severe complications following pancreaticoduodenectomy [2] and although mortality improved over time from 8% during 1990-1999 era to 2% during 2000- 2007 era [1], the high associated morbidity underscores the importance of a robust pre-operative evaluation prior to considering definitive surgical therapy.
We reviewed the literature to identify all reported preoperative worrisome features and markers of malignancy in PCL and studied the pre-operative imaging in our small cohort in relation to the following features:
• Presence or absence of Septations
• Main pancreatic duct features (Dilatation of pancreatic duct)
• Size of PCL (>3 cm)
• Proximal or distal location of the cyst (i.e. Head, body or tail)
• Solid components (present or absent)
• Shape/Border (Oval, round, branching or single)
• Calcification
• Signal intensity (T1 or T2 attenuated lesion, High or Low signal intensity)
• Biliary tract involvement
• Solitary, unilocular or multilocular
• Wall Worrying features (thick or thin, regular or irregular, presence or absence of mural nodules)
• Intra-cyst fluid CEA, CA19-9 and amylase levels
We carried out a systematic literature review to identify the relevant variables that are utilized to predict the nature of pancreatic cystic lesions (PCL) and aid therapeutic planning. This was followed by designing a template to capture all these variables for the resected lesions from two centres. We collated clinico-pathological, biochemical data, pre-operative CT, MRI, EUS, PET CT, FNA analysis whenever they are performed and correlated with the post-operative histology result. We identified 64 patients from medical records who were evaluated to a variable degree for the presence of the PCL; 27 of those underwent EUS-FNA as part of the pre-operative evaluation of the nature of the cyst as an adjunct to the other radiological investigations and 15 underwent surgical resection. Pooled data was analysed using STATA 14.
Sixty-four patients with pre-operative diagnosis of pancreatic cystic lesions were identified. Fifteen patients underwent surgical intervention. The final histology of these cysts was compared with preoperative predicted nature based on the various investigation modalities (Table 1).

Of the fifteen resected cases, 2 turned out to be serous and one confirmed to be mucinous cyst adenoma; two had IPMN (Intraductal Papillary mucinous neoplasm) while one case was VHL (Von Hippel – Lindau syndrome). Cystadenocarcinoma’s was found in 5 cases, endocrine neoplasm in 1, pseudocyst in 2 of the resected cases and histology result of one of the resected cases could not be found.
Morphological characteristics of the 15 patients (Table 2) suggests that the majority of these lesions were either multicystic and or multilocular; with or without thickening of wall and with or without septations.

We noted calcification in two cases and duct dilatation in 7 others; and it was interesting to note that most of our small resected cohort did not have any cyst related solid components despite the malignant nature of some of them. Various modalities were utilized in the pre-operative evaluation of the 15 resected cases (Table 3) noting the tendency towards combining modalities.
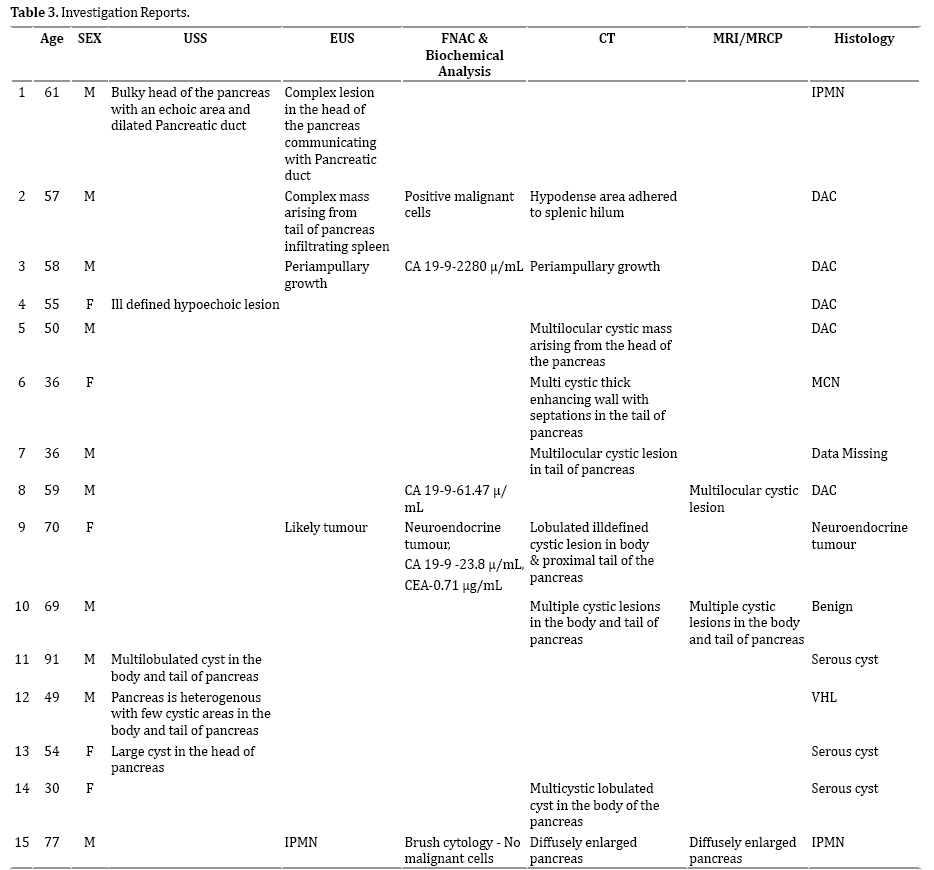
Twenty-seven cases underwent EUS- FNA as an adjunct to the radiological assessment to evaluate the nature of these PCLs; and both cytological and biochemical analyses were carried out on the intra-cystic aspirate (Table 4). The intra-cystic CEA levels recorded a mean of 667.97 in the tested group with a wide standard deviation of 1934.38 (results from STATA 14 are attached as a supplement).

The small size of our retrospective cohort and lack of homogeneity for the various modalities of investigations utilized for evaluation of such diverse pathology (cystic lesions of pancreas) precluded any meaningful analysis of the diagnostic accuracy of each individual modality.
Distinguishing benign from malignant or premalignant PCL is essential when formulating the surgical therapeutic strategy and lack of well-defined pre-operative predictability criteria makes therapeutic planning challenging. Pancreatic cystic lesions can generally be divided into 4 main categories:
I. Benign cystic lesions: This includes pseudocyst, serous cyst, retention cysts, parasitic cysts and pancreatic abscess.
II. Premalignant conditions: This includes intra-ductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN).
There are no reliable predictors of malignancy for patients with main duct MD-IPMN, although several studies have described clinical and radiological features that are more common in MD-IPMN carcinoma such as jaundice, recent onset or deterioration of diabetes mellitus (Saliva et al.2004). Other common symptoms include weight loss, abdominal pain and steatorrhea. Radiological suspicious findings for malignant transformation in MD-IPMN are mural nodules or associated mass, enhancing cyst wall and a maximum main duct of > 10 mm (Manfredi et al. 2009, Sahani et al.2006). On the other hand, branch duct BD-IPMN without mural nodules (MNs) have a low risk of progression and malignant transformation and hence the suitability of this group for non-surgical management and safety of medium to long interval surveillance. Although a cyst size >3 cm was previously thought to be one of the predictors of malignancy and hence the recommendation for resection in the first consensus guidelines (Tanaka et al. 2006); the 2012 revised guidelines are more reserved and suggests that BD-IPMN > 3 cm without any signs of other risk factors (i.e. mural nodules) may be observed without immediate resection. We retrospectively studied the radiological features in our cohort to test the correlation between them and the final histology as proof-of-principle exercise aiming at highlighting the high risk lesions based on the pre-operative features. Three of the 15 resected cases in our cohort who were evaluated using combined assessment were accurately identified and predicted as IPMN; two of them underwent curative resection confirming the final histology to be IPMN in both while the third patient declined surgery but was confirmed by FNA.
Mucinous cystic neoplasms (MCN) are pre-cancerous and almost always located in the pancreatic body/tail (99.4%) in female patients (98.1%) Yamao et al.2011).. In our study we encountered one case of MCN, which was identified correctly by combined assessment, and patient had a curative resection for this lesion.
III: Malignant Pancreatic Conditions: This includes ductal adenocarcinoma and malignant mucinous cystic neoplasm.
IV: Others (neuroendocrine tumor & VHL)
A. MDCT (Multi-Detector Computed Tomography)
According to Chalian et al. [6], the presence of thickened irregular walls/septa on MDCT correlated well with malignancy. In contrast, presence of thickened irregular walls/septa on MRCP and intramural nodules on EUS reported no correlation with malignancy. Furthermore, attenuation measurement may occasionally help in differentiating pseudocysts from unilocular mucincontaining simple cysts of the pancreas on CT images. Attenuation in pseudo cyst was reported as 18.9 HU (95%CI: 15-22.7 HU), MCN as 13 HU (95%CI: 10.6-15.5 HU) and IPMN as 11.4 HU (95%CI: 8.8-14.1 HU) [6].
In a prospective study conducted by Sahani et al. in 2011 relating to inter-reporter variation in description of MDCT features, the radiological accuracy (reader 1 and reader 2) for stratifying lesions into mucinous and nonmucinous subtypes was reported to be 85% and 82%; and for recognizing cysts with aggressive biology was reported to be 86% and 85%, respectively. The predictive power of MDCT was reported as superior for lesions >30 mm and for non-mucinous lesions. Features favoring aggressive biology were reported as main pancreatic duct dilation >10 mm (P<0.0001), mural nodule (P<0.0001), main-duct intraductal papillary mucinous neoplasm (P<0.0001), and advanced age (P=0.0001). Sensitivity of detecting morphologic features was reported as higher with the dual-phase pancreatic protocol CT [7].
In our study CT was done in 41 patients 7 were reported as suspicious for malignancy and 3 were thought to be cystic degeneration of solid tumors.
Apparent diffusion coefficient (ADC) measurements from diffusion-weighted imaging (DWI) can characterize and may predict the malignant potential of cystic pancreatic lesions. ADC values may be helpful in deciding the malignant potential of IPMN. However, they are not useful in differentiating malignant from benign lesions or for characterizing PCL [8].
In our study MRI was done in 8 patients; 3 were suspicious for malignancy (1 was not picked up by CT) and 1 was cystic degeneration of solid tumor.
Cyst size over 30 mm, mural nodule over 6 mm, irregular thick septa, dilatation of the MPD, and the presence of soft tissue mass may be helpful factors in predicting malignancy [10].
A forward-viewing echo endoscope that allows target sites to be punctured more perpendicularly with minimal effort, can be used for diagnostic EUS-FNA and this may be advantageous, depending on the site of target lesions [11] .
The incidence of infectious complications after EUSFNA of pancreatic cystic lesions, with or without antibiotic prophylaxis, appears very low [12].
EUS with cyst fluid analysis can be successfully used to rule out pancreatic neoplasms and to follow-up incidentally discovered PCL [13]. Age and EUS appearance independently predict surgery in patients with pancreatic cysts referred for EUS. The “perceived need for EUS-CFA (cyst fluid analysis) also predicts surgery, but not the EUSCFA results. The clinical value of EUS-CFA requires further research [14].
The cytological diagnoses were correlated with cyst fluid carcinoembryonic antigen (CEA) level and subsequent histologic diagnoses [15].
In our study EUS was performed in 38 patients; 8 were suspected to be malignant based on EUS morphological criteria (2 were not picked up by CT). Three of 8 patients had neoplastic cells on FNAC; 2 had FNAC results suspicious for neoplasm, and 31 were reported to have benign FNAC.
Cyst fluid can be further analysed after aspiration for cytology, viscosity, extracellular mucin, other tumour markers (CEA, CA 19-9,CA 15-3, CA 72-4, etc.), enzymes (amylase, lipase), as well as DNA analysis of DNA quality/ content or mutational analysis to study allelic imbalance/ LOH (loss of heterozygosity) and K-Ras mutations [14].
Review of the literature suggests that CA 72-4 cyst fluid levels were found to be significantly higher in mucinous cystic tumors (P<0.005), with 80% sensitivity and 95% specificity in detecting mucinous or malignant cysts. A subsequent study found that a CA 72-4 level over 40 U/mL had a 63% sensitivity and 98% specificity for distinguishing mucinous cyst adenomas and cyst adenocarcinomas from serous cyst adenomas and pseudocysts. Intra-cystic CEA level of >400 ng/mL is reported to have 57% sensitivity and 100% specificity for distinguishing mucinous tumours and cyst adenocarcinomas from pseudocyst, ( Bhutani et al. 2011). Cytological identification of extracellular mucin and CEA are thus considered predictors of MCN and malignant MCN, as recently proven by a multivariate analysis in 43 patients, which suggested CEA threshold levels >300 ng/ mL (P=0.0007) and identification of mucin (P<0.001) as reliable predictors [14]. It is also noted that CEA >=6000 ng/mL differentiates malignant from benign MCN.
In a study by Frossard et al. a CA 19-9 value greater than 50,000 U/mL in the cyst fluid had 15% sensitivity and 81% specificity to distinguish mucinous cysts from other cystic lesions.
In our study we noticed elevation of intra-cystic CEA levels to >300 ng/mL in 10 cases and CA 19-9 results of >2000 IU/mL in 4.
None of the available pre-operative diagnostic modalities can reliably predict the nature of non-metastatic pancreatic cystic lesions and it is our view that further research to evaluate the utility of the following diagnostic adjuncts are necessary:
1) Proteomic profiling of pancreatic cystic lesions [16], (Various mucin proteins expression in the cyst fluid such as MUC 1, MUC2, MUC 5 & MUC 7).
2) GNAS mutations were reported to be present in 66% of IPMNs and that either KRAS or GNAS mutations could be identified in 96% [17].
3) RNA can be extracted from samples obtained from EUS-FNA. MUC7 from samples could serve as a potential biological marker to identify malignant lesions, especially pancreatic adenocarcinoma [18].
No single test is able to predict the nature or behavior of pancreatic cystic lesions. The differences noted on specialist imaging can be very subtle and demand specialist interpretive skills. A panel of pre-operative testing and review at specialist MDT is mandatory for all such cases. Study of our cohort highlights the importance of the preoperative combined clinical, radiological (CT/PET/MRI/ EUS) and the necessity of FNAC and biochemical analysis prior to therapeutic planning. Sensitivity and specificity may be increased by genetic testing of the cyst aspirate material. MRI/MRCP is superior to MDCT in the detection and characterisation of IPMN. Branch duct IPMN (BDIPMN) is relatively common, image findings of BD-IPMN may overlap those of other pancreatic cysts and when MDCT or MRI/MRCP fail to demonstrate communication with the main pancreatic duct (MPD), the differential diagnosis between BD-IPMN and oligocystic serous cystic neoplasm (SCN) may be difficult. MDCT can compensate for MRI in cases where the image quality is degraded, is a reliable modality in evaluating preoperative vascular anatomy and curved planar reformation images can also be created along the course of the MPD making it visually demonstrable. MRI/MRCP however remains to be the preferred modality for follow-up imaging as it lacks ionizing radiation. Determining whether a pancreatic cyst is mucinous or non-mucinous, benign or malignant are the key clinical questions that drive patient management; and analysis of the pancreatic cyst fluid is a vital component of the multimodal approach for preoperative evaluation.
We are proposing a flow chart (Figure 1), which reflects our experience including our interpretation of current knowledge in characterisation and management of PCL but this should be applied in the context of a specialist pancreatic MDT.
The authors had no conflicts of interest