- (2014) Volume 15, Issue 4
Saira Burney1, Khadija Irfan Khawaja1, Muhammad Wasif Saif2, Faisal Masud3
1Department of Endocrinology & Metabolism, Services Hospital. Lahore, Pakistan
2Tufts Medical Center and Tufts Cancer Center. Boston, MA, USA
3King Edward Medical University. Lahore, Pakistan
Pancreatic cancer, despite being a relatively less commonly occurring cancer is among the deadliest ones, leading to a grave prognosis. Surgery stands as the mainstay of treatment of pancreatic cancer but is an option in less than 15% patients owing to the late presentation of the tumor. Chemotherapy offers an important part of treatment but can adversely affect the quality of life because of devastating side effects and has limited survival benefit. Unavailability of effective and less toxic treatment options for pancreatic cancer has prompted the search for new treatment strategies. One such drug being considered for its potential anti-neoplastic role is the time-tested and widely used oral hypoglycemic drug, metformin. Metformin is proposed to target metabolic pathways involved in tumorigenesis, specifically the AMPK- mTOR complex. Epidemiological evidence is mounting in favor of its role in various cancers both for treatment and prophylaxis. Herein, we aim to summarize the epidemiological data on metformin as a potential anti-cancer drug in various cancers followed by a look at some of the abstracts relating to this topic that were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting 2014.
Metformin; Pancreatic Neoplasms; Review; Therapeutics
ASCO: American Society of Clinical Oncology
Worldwide, cancer has a huge health impact and is showing increasing trends with time. Adding to this burden of cancer is diabetes, which, together with underlying insulin resistance is now considered a risk factor for several malignancies [1]. The mechanisms proposed to explain the connection between diabetes and cancer includes hyperinsulinemia, hyperglycemia and IGF pathway stimulation [2]. There is considerable epidemiological evidence to support the bidirectional and time-dependent relationship between pancreatic cancer and diabetes; long-standing diabetes has been shown to double the risk for pancreatic cancer while new-onset diabetes can be an early manifestation of the disease [3].
Metformin is an oral biguanide that forms the cornerstone of management of type 2 diabetes both as monotherapy and in combination therapy. It mediates its glucoselowering effect principally through suppressing hepatic gluconeogenesis, which in turn reduces hyperinsulinemia, and also by enhancing peripheral insulin sensitivity and increasing glucose uptake and utilization [4]. These effects are achieved by the action of metformin at a cellular level through the activation of an intracellular hepatic enzyme, AMPK that is involved in regulation of several critical pathways including energy metabolism and cell proliferation [4].
The recent years have seen epidemiological evidence building up to support the anti-proliferative role of metformin in cancer. There are many studies that have reported a reduced incidence of various cancers as well as cancer-related mortality in diabetic patients taking metformin [5]. Table 1 shows a summary of studies that have examined the use of metformin in various cancers and reported a reduced risk in diabetic patients with metformin [6-7].

Several mechanisms are proposed to explain the potential anti-neoplastic action of metformin. The insulin-IGF-1 signaling pathway appears to be mitogenic promoting cell proliferation and tumor formation. Metformin ameliorates hyperinsulinemia both by reducing hepatic glucose output and by increasing peripheral insulin sensitivity. The main anti-cancer mechanism is attributed to activation of cellular AMPK, which in turn inhibits mTOR pathway via LKB1 thereby suppressing cell growth and tumor formation [4, 8].
The protective effect of metformin against cancers also extends to pancreatic cancer. In a prospective case control study in 1836 patients, Li et al. reported a significant reduction in risk of pancreatic cancer (OR: 0.38, 95% CI: 0.21-0.69, P=0.001) in patients on long-term treatment with metformin compared to those not taking metformin [9]. In other studies, use of metformin in type 2 diabetic patients has been reported to reduce the risk of gastrointestinal malignancies including pancreatic cancer in type 2 diabetic patients [10]. Similarly, the results of a large retrospective cohort study by Currie et al. showed that diabetic patients treated with metformin alone had the lowest risk of cancer of pancreas and colon in comparison to patients treated with sulphonylureas and insulin [11].
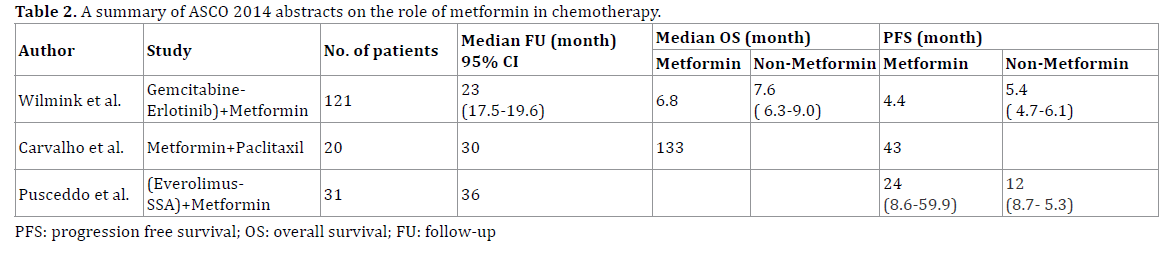
A summary of ASCO 2014 abstracts on the role of metformin in chemotherapy is shown in Table 2.
Gemcitabine, Erlotinib, and Metformin in Patients with Locally Advanced or Metastatic Pancreatic Cancer
In this context, three studies were presented at the American Society of Clinical Oncology Annual Meeting 2014 focusing on the possible role of metformin in the treatment of pancreatic tumors, both solid and neuroendocrine. Wilmink et al. (Abstract #4021) evaluated the efficacy of metformin as add-on therapy with gemcitabine and erolitinib in advanced pancreatic cancer [12]. 121 patients with advanced pancreatic cancer were randomly assigned to receive gemcitabine and erlotinib plus either metformin in incremental doses based on tolerance from 500mg twice daily for one week to 1000mg twice daily or placebo. The objective response rate was the same in both treatment and placebo group. However, survival at 6 months, which was the primary end-point, while overall survival and progression-free survival were secondary end-points both were higher in the placebo group compared with the treatment group. The investigators concluded that the addition of metformin to chemotherapy treatment with gemcitabine and erlotinib was well tolerated but was not associated with statistically significant improvement in survival, the primary end-point of the study, compared with placebo. Combination therapy had an acceptable safety profile.
Metformin and Paclitaxel for Patients with Gemcitabine- Refractory Advanced Adenocarcinoma of the Pancreas
The second study of interest was reported by Carvalho et al. (Abstract #e15196) [13]. This trial targeted patients with advanced gemcitabine refractory pancreatic adenocarcinoma, which usually has a median survival measured in days. This phase II trial was motivated by the encouraging results of metformin as an anti-tumor agent in pre-clinical studies. The trial objective was to determine if the addition of metformin to a monthly dose of paclitaxel could favorably impact survival outcomes in patients whose cancer had been refractory to the standard gemcitabinebased chemotherapy. As this was an uncontrolled trial, a comparative analysis was not possible. Instead they chose an arbitrary criterion of 8 weeks’ progression free survival, in half of 23 patients (who were to be recruited in the first stage), as the primary end point.
As only 6 patients (31.6%) achieved this, the trial was not carried to the second stage of recruitment. The primary end-point of disease control at 8 weeks could not be met in this trial, as the mean progression free survival in these patients was only 43 days. Hence they concluded that metformin was not beneficial as add-on therapy to paclitaxel in gemcitabine-refractory pancreatic adenocarcinoma. Furthermore, the combination was also poorly tolerated, warranting against further investigation.
Impact of Metformin on Progression-Free Survival of Patients with Advanced Pancreatic Well-Differentiated Neuroendocrine Tumor Receiving Everolimus plus Somatostatin Analog
Finally, the third study was a retrospective analysis by Pusceddo et al. (Abstract #e15172) [14]. This was a retrospective study, which included patients with welldifferentiated pancreatic neuroendocrine tumors, who were being treated with everolimus and somatostatin analog combination therapy. 31 age and gender-matched patients were found in the 3-year study period. The patients were stratified based upon their diabetes status, and treatment with either insulin or metformin, into three groups: normoglycemic controls (n=19), insulin-treated diabetic patients (n=6) and metformin-treated diabetic patients (n=6).
The result showed that the median progression-free survival in the diabetic patients was twice that observed in the control group. The study was reported while treatment of the metformin group was still ongoing, although four patients had reached a mean progression free survival (mPFS) duration of 19.5 months, as opposed to mPFS of 12.2 months for the patients on insulin and 12 months for the control group. However, the retrospective nature of the study placed limitations on the significance of these findings. The treatment allocation in the diabetic group could be influenced by the severity of diabetes or even the tumor, as insulin treatment is often the choice in sicker patients than those who receive oral agents such as metformin. Furthermore, the number of patients reported in both the diabetes treatment arms was too small to allow for any significant difference to be noted. Despite these limitations, neuroendocrine tumors are rare and the data reported here is of importance, and should provide an incentive for larger studies.
Research indicates a potential role of metformin as adjuvant to chemotherapy in various cancers. In diabetic patients diagnosed with cancer, simultaneous treatment with metformin has shown to improve response to chemotherapy [15]. Metformin co-treatment may also allow for a dose reduction of chemotherapeutic agents in patients undergoing cancer treatment thereby reducing the side effects of chemotherapy [15].
The clinical trials discussed in this review provide conflicting evidence on the use of metformin in advanced pancreatic carcinoma. The study conducted by Wilmink et al. was a large trial, with a long follow up in the context of advanced/metastatic PAC, where survival is usually measured in months. It aimed to test an important preclinical finding in a clinical setting, whether metformin addition to standard gemcitabine-based therapy would increase PFS in advanced PAC. Although preclinical evidence is supportive, the results of this trial were disappointing as the overall and PFS survival in the placebo group was higher than the metformin arm, although the difference was not statistically significant. Failure to achieve statistical significance may be explained by a small sample size or a high tumor burden. The results of this particular analysis are similar to those of a retrospective study by Sadeghi et al. in which metformin therapy was associated with significantly increased survival in patients with nonmetastatic disease only [16]. Gemcitabine-based regimens have been the established first-line treatment for advanced pancreatic cancer but drug resistance to gemcitabine is increasingly being encountered necessitating the need for second-line agents. In this respect too, metforminpaclitaxel combination gave discouraging results and was poorly tolerated. However, the addition of metformin to everolimus-SSA chemotherapy for pNET showed some promise regarding the adjuvant role of metformin despite the very small sample size and with only partial results available. The increased duration of survival was of importance, and may have implications for future selection of treatment for similar patients.
Further studies in large populations are needed as are outcome studies specifically designed and powered to test for the effect of metformin on outcomes. It may be early days yet but a whole new area of research has opened up and looking into the promising role of metformin in cancer treatment.
The authors have no potential conflicts of interest.