- (2008) Volume 9, Issue 3
Hamid Saadati, Muhammad Wasif Saif
Yale University School of Medicine. New Haven, CT, USA
Received January 30th, 2008 - Accepted February 15th, 2008
Dear Sir:
There have been constant debates regarding the issues of chemotherapy use and dosing in setting of liver insufficiency in patients with solid malignancies. Many chemotherapeutic agents are metabolized by the liver, and it is also relatively common for advanced cancer patients to have liver dysfunction secondary to metastatic disease. Some literature reviews the use of specific cytotoxic drugs in such patients [1, 2] but data are still lacking for most agents. Hence, there are no formal dosing recommendations. Furthermore, most patients with hepatic insufficiency are excluded from clinical trials and case review series, perpetuating the lack of such information. This situation is further complexed when dealing with a patient with pancreatic cancer, where options are extremely limited and liver dysfunction is very common.
Capecitabine is an oral, tumor-selective fluoropyrimidine carbamate that is preferentially converted to active 5-fluorouracil (5-FU) within the tumor by a three-step enzymatic conversion [3, 4, 5]. Due to ease of administration and better safety profile, capecitabine is being used with increasing frequency as an alternative to infusional 5-FU therapy for metastatic gastrointestinal malignancies [6]. We recently reported a series of three patients suffering from gastrointestinal malignancies and associated mild-moderate liver insufficiency who received capecitabine for their metastatic disease [7].
In this letter, we report another case of a patient with pancreatic cancer and liver insufficiency who received capecitabine as a palliative chemotherapy.
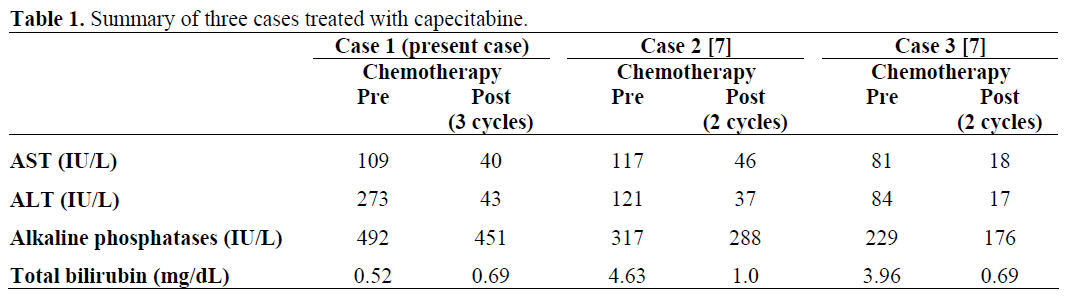
Our patient is a 48-year-old male who presented with progressively worsening of abdominal pain prompting an evaluation by his gastroenterologist. His abdominal CT scan showed pancreatic mass involving the neck and body of the pancreas with encasement of the celiac axis. Extensive retroperitoneal lymph node metastases and multiple pulmonary nodules consistent with metastasis were also noted. Fine needle aspiration of the pancreas confirmed poorly differentiated adenocarcinoma. Patient was initially treated with gemcitabine-based chemotherapy. However, this regimen was discontinued after a staging CT scan confirmed progression of disease. He then began treatment with gemcitabine plus oxaliplatin but he developed severe transaminitis with cycle one (AST 145 IU/L, reference range: 0-35 IU/L; ALT 242 IU/L, reference range: 0-35 IU/L) and normal total bilirubin (0.42 mg/dL; reference range: 0-1.20 mg/dL). He received oxaliplatin alone during cycle 2. Therapy was then changed to single agent gemcitabine due to further elevation in ALT (328 IU/L). In addition to abnormal liver function tests, his chemotherapy was also complicated by grade 4 neutropenia (absolute neutrophil count 477/mm3, reference range: 1,500-8,000/mm3). CT scan indicated progression of pulmonary metastases. Due to persistent elevation of AST and ALT, treatment options were very limited due to concern about safety. After a detailed discussion with the patient, capecitabine 1,500 mg po twice a day with one week on and one week off (7/7 schedule) was administered. He tolerated capecitabine well with significant improvement in transaminases (Figure 1) with minimal toxicity. Unfortunately, his restaging CT scan after three cycles showed disease progression with growing of peritoneal and liver metastases as well as new liver metastases.
In addition to our two earlier reports of pancreatic cancer patients with liver dysfunction [7], this case further suggests that capecitabine may be safely used to treat an advanced pancreatic cancer with associated hepatic insufficiency (Table 1). Generally, the pharmacokinetics of capecitabine are not affected in patients with mild to moderate hepatic dysfunction, but there are no data available for patients with severe hyperbilirubinemia. Our clinical experience also corresponds to the findings of Twelves et al. [8] who reported that impaired hepatic function had no clinically significant influence on the pharmacokinetics of capecitabine and its metabolites. It is important to remember that the liver dysfunction in this patient was secondary to his malignancy. Akin to other two cases of pancreatic cancer, our patient also showed clinical response even at a reduced dose of capecitabine [9]. This finding is particularly interesting, as it shows that the efficacy of capecitabine was not necessarily compromised at the reduced doses chosen for this patient. Clinical trials of low-dose capecitabine in such patients are therefore warranted. In addition, we used a novel schedule of capecitabine [10]. Preclinical studies in human tumor xenografts indicate that inhibition of tumor growth depends on the total dose of capecitabine but not necessarily on its administration schedule. This was tested in a randomized phase II study suggesting that short intermittent schedules have equal efficacy to more protracted administration. This hypothesis was tested further by Scheithauer et al., in a single center, European phase I/II study in advanced colorectal cancer. Patients received oxaliplatin at a fixed dose of 85 mg/m2 every 2 weeks combined with escalating doses of capecitabine [11]. In this regimen, the dose intensity of capecitabine was from 105% to 131% compared with the conventional capecitabine administration schedule of days 1-14 every 3 weeks (21 days). Gastrointestinal toxicities, predominantly diarrhea, were the principle toxicities seen in the study. It is noteworthy that cohort expansion was necessary in all but the first dose level where no dose limiting toxicities were seen. Additionally, the toxicity profile began to shift beyond 1,500 mg/m2 twice-daily from nausea and vomiting to diarrhea at higher doses. Despite the shift toward more severe and clinically significant toxicities being seen at the higher dose levels and the need for cohort expansion, the dose recommended for further testing was capecitabine 1,750 mg/m2 orally twice-daily for 7 days followed by 7 days without capecitabine in repeated 2-week cycles. Interestingly, efficacy results were encouraging with a response rate of 50 % and a median progression-free survival of almost 9 months. Our case also emphasize that capecitabine can be used in a range of dosing schedules and intensities and future studies should focus on a dose intense weekly schedule in patients with liver dysfunction.
The authors have no potential conflicts of interest