Case Report - (2018) Volume 19, Issue 6
1Department of Internal Medicine/Emergency Medicine, University of Illinois Hospital & Health Sciences System, Chicago, Illinois, United States of America
2Department of Medicine, University of Illinois Hospital & Health Sciences System, Chicago, Illinois, United States of America
Received September 22nd, 2018 - Accepted November 03rd, 2018
Context There are anatomic variations of pancreatic ducts with ansa pancreatica being a rare anatomic variant where the duct of Santorini forms a loop or S-shape. It has been postulated that ansa pancreatica predisposes people to acute pancreatitis. We report a case of ansa pancreatica, review the literature and examine its relation to acute pancreatitis. Case report A sixty-eight-year-old male, with a previous episode of acute pancreatitis, presented to the emergency department with epigastric pain, was found to have a lipase of 5,399 U/L and a normal right upper quadrant ultrasound. Metabolic workup, triglycerides, and IgG4 levels were all within normal limits. There were no other medical problems or medication usage which would predispose the patient to acute pancreatitis. A magnetic resonance cholangiopancreatography was performed which showed a dilated pancreatic duct to 6 mm in the head and body and also evidence of ansa pancreatica. Following resolution of the acute episode, an endoscopic retrograde cholangiopancreatography revealed moderate diffuse dilation in the ventral pancreatic duct with an ansa loop in the pancreatic duct head, indicating ansa pancreatica. A sphincterotomy of the major papilla was performed and a temporary stent was placed. The patient has had no additional acute pancreatitis episodes. Conclusion Findings from our case and literature review show that ansa pancreatica increases one’s risk of acute pancreatitis. A detailed investigation in cases of idiopathic acute pancreatitis can play a key role in early identification and treatment of ansa pancreatica and can improve clinical outcomes.
Cholangiopancreatography, Endoscopic Retrograde; Pancreas; Pancreatic Ducts; Pancreatitis
AP acute pancreatitis; ED emergency department; ERCP endoscopic retrograde cholangiopancreatography; EUS endoscopic ultrasound; IV intravenous; MMPD meandering main pancreatic duct; MRCP magnetic resonance cholangiopancreatography; PD pancreatic duct
Acute pancreatitis (AP), an inflammatory disease of the pancreas, is a common cause of gastrointestinal hospitalizations, and AP results in significant health-care burden [1]. Gallstones, alcohol intake and smoking are well known causes of AP [2]. Variations in pancreatic duct (PD) anatomy have also been shown to play a role in some cases of AP. Typically, the downstream pancreatic duct system within the head is made up of the ducts of Wirsung and Santorini (the accessory pancreatic duct). There are many different types and anatomic variations of the pancreatic ducts with ansa pancreatica being a rare anatomic variant. Ansa pancreatica has been described in two forms [3]. The first is where the duct of Santorini forms an S-shape (Figure 1) en route to the duct of Wirsung [3, 4]. In the second form, a looping branch is seen within the duct of Wirsung as it joins the duct of Santorini (Figure 1) [3]. The contribution of this anatomic variant to AP has been still debated [5]. Here, we report a case of ansa pancreatica resulting in AP, discuss underlying pathophysiology and review the literature for this anatomic variant.
Patient consent was obtained to allow for discussion of the following case. A sixty-eight-year-old male with a past medical history of one prior episode of AP of unknown etiology, gastric ulcer, Helicobacter pylori infection treated with triple therapy, and benign prostatic hypertrophy status post prostatic resection presented with epigastric abdominal pain. One month prior to this presentation the patient had his first documented episode of AP. A CT scan at that time had shown a mildly dilated PD of 4 mm in the head of pancreas with edematous interstitial pancreatitis in the tail of the pancreas. The patient improved after a brief period of bowel rest and adequate hydration with intravenous (IV) fluids. He was discharged home and was scheduled for an outpatient endoscopic ultrasound (EUS). The EUS showed normal parenchyma in the pancreatic head, neck, body, tail, and uncinate process, and it also revealed a dilated PD in the head, body, and tail of the pancreas which measured up to 4.2 mm. A magnetic resonance cholangiopancreatography (MRCP) was planned, but in the meantime the patient experienced his second episode of abdominal pain.
His pain was located in epigastrium, associated with anorexia and started one day prior to arrival to the emergency department (ED). He reported drinking one shot of liquor at a sporting event five days prior but denied any considerable or chronic alcohol intake. He was found to have a lipase of 5,399 U/L and a normal right upper quadrant ultrasound. The patient was also found to have a normal calcium, triglyceride (102 mg/dl), and IgG4 level (45 mg/dl). He had no evidence of infection, and he was not on any new or high-risk medications. A MRCP was performed which showed a dilated PD to 6 mm in the head and body and evidence of ansa pancreatica (Figures 2, 3). The patient was then discharged home after standard treatment and significant improvement in symptoms. His outpatient ERCP confirmed moderate diffuse dilation in the ventral pancreatic duct with an ansa loop in the PD (Duct of Wirsung) in the head of the pancreas (Figure 4). A guidewire was passed into the body of the pancreatic duct from the major papilla. The loop appeared fixed, and no catheter could be passed through the loop over the wire. A sphincterotomy of the major papilla was performed and 5 French by 3 cm plastic stent was placed in the PD abutting the downstream edge of the ansa loop (Figure 5). The patient was discharged and the PD stent was confirmed to have migrated spontaneously on abdominal film 1 week later. The patient was discharged with complete resolution of abdominal pain and without any additional episodes of pancreatitis.
In a normal pancreatic ductal system the majority of drainage from the pancreas comes from the duct of Wirsung through the major papilla with a much smaller proportion of the drainage occurring through the accessory pancreatic duct out of the minor papilla [5]. These two ductal systems form separately in embryological development, rotate, and then fuse around 6-8 weeks of gestation. This process can go awry causing many different types of anatomic variants with one of those being ansa pancreatica [5]. Ansa pancreatica was first described in the literature by Dawson and Langman in 1961 [6]. They described this variant as “obliteration of the accessory pancreatic duct” [6]. It typically connects with the duct of Wirsung but in this case, this connection is replaced by a different arched duct that originates from the main pancreatic duct and forms a loop that terminates in the minor papilla [5, 6]. However, the study that first described ansa pancreatica found that in about two-thirds of patients studied with ansa pancreatica, the communication into the duodenum was not patent and thus did not allow for pancreatic drainage to flow freely [6].
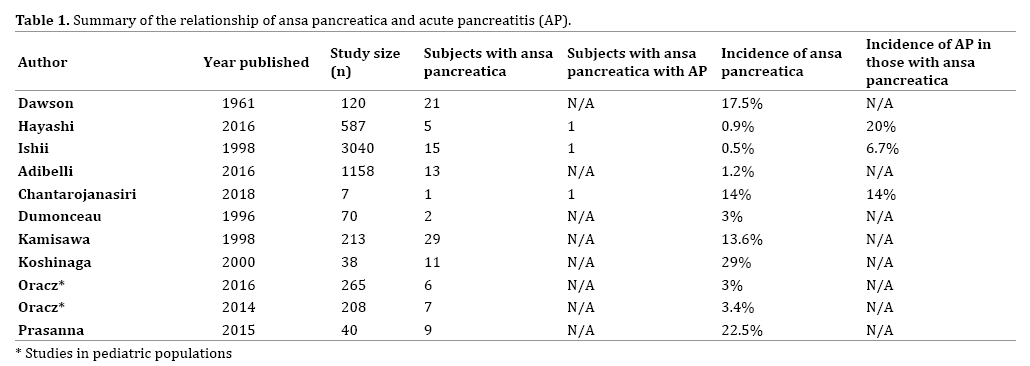
Ansa pancreatica is not well studied in the literature, and the prevalence is not well established. After a thorough literature search, only three articles were found that commented on the rate of occurrence of ansa pancreatica in relation to acute pancreatitis (Table 1). Hayashi et al. performed a study in Japan that consisted of a communitybased cohort of 587 patients who attended a paid health checkup where among other tests an MRCP was performed on all of the subjects [7]. They found that 0.85% of patients had ansa pancreatica [7]. These subjects were almost entirely Japanese, so this may not apply to the general population in other regions of the world. Also, MRCP may underestimate the actual incidence of ansa pancreatica. Ishii et al. looked at all of the ERCPs done at their institution in Tokyo over a 24-year period, and they found that out of the 3040 patients that had ERCPs only 15 of these had ansa pancreatica with a rate of 0.5% [8]. Another study performed in Turkey by Adibelli et al. retrospectively looked at 1312 patients who had a MRCP and found that 1.2% of this population had ansa pancreatica [9].
The patient presented above has a ductal abnormality which is generally considered a variant of ansa pancreatica, though there is a lack of uniform agreement in terminology. Manuscripts from Europe and Asia have described two variants of ansa pancreatica: the classic loop of the duct of Santorini terminating at the minor papilla, or the looping branch of the duct of Wirsung as it joins the duct of Santorini [3, 10]. In contrast, Gonoi et al. included the latter abnormality within a group named “meandering main pancreatic duct (MMPD),” likely as the abnormality is present within the duct of Wirsung [11]. Of note, the MMPD was also felt to predispose one to acute pancreatitis [11]. The lack of agreement in terminology is likely due to the rarity of this ductal variant.
Because of the often poor communication into the duodenum and the thought that the areas emptied by the ansa branch have inadequate drainage (due to anatomic positioning of the duct), it has been postulated that ansa pancreatica predisposes patients to AP [5, 6]. Both Kamisawa et al. and Tabata et al. showed that a decrease in patency in the accessory pancreatic duct leads to an increase frequency of pancreatitis [12, 13]. This supports the idea that the anatomic variations are clinically relevant. The patency of the accessory pancreatic duct impacts its ability to empty pancreatic secretions, which can increase intraductal pressure and affect the rate of pancreatitis [6, 12]. As shown by Dawson and Langman a significant amount of those with ansa pancreatica did not have patent pancreatic accessory ducts [6].
AP is diagnosed by having two of the three following criteria: a serum amylase or lipase greater than three times the upper limit of normal, abdominal pain consistent with AP, and findings on abdominal imaging consistent with AP [14, 15]. The mainstay of treatment is adequate fluid resuscitation, especially within the first 24 hours [14]. Lactated Ringer’s solution is typically recommended, and most recent guidelines from American Gastroenterological Association recommend using goal-directed therapy for fluid resuscitation [14, 15]. It is recommended that in mild AP oral feedings should be started as soon as can be tolerated, and in severe AP enteral nutrition is preferred over parenteral nutrition as this can decrease infection risk [14, 15]. If there is concern for choledocholithiasis with acute cholangitis, then an ERCP should be performed [14]. However, if there is no concern for cholangitis but choledocholithiasis is still suspected, MRCP or EUS should be performed over ERCP [14]. The routine use of antibiotics is not recommended in acute pancreatitis, even in sterile necrosis [14, 15]. However, sometimes the necrosis can be infected, and antibiotics can be effective in this situation [14].
The treatment for AP as described above would also apply to AP in someone with ansa pancreatica. Since MRI has become more sophisticated it is now easier to detect the ansa loop using MRCP, but ERCP is still the gold standard [7]. When looking at a MRCP, it can be easier to see the ansa loop if the 3D-T2 weighted images are used. In regards to treatment specific to AP in ansa pancreatica patients, it is important to remember the minor papilla was found to be non-patent in over 60% of these patients as found by Kamisawa and Dawson [6, 12]. Treatment strategies are not well described in the literature, but it is reasonable to postulate that because of the above findings described by Kamisawa and Dawson, that relieving downstream pressure may be of benefit [6, 12]. This was the treatment strategy followed in the case described above in which a sphincterotomy of the major papilla was performed along with a stent placement downstream to the ansa loop, and this resulted in the resolution of his AP.
After a thorough literature review, we identified three case reports about idiopathic AP being caused by ansa pancreatica [5, 10, 16]. In the study performed by Hayashi et al. described above in addition to the community group, the researchers also looked at a cohort of 73 patients with acute pancreatitis [7, 17, 18, 19]. The study found that patients with recurrent acute pancreatitis had a higher rate of ansa pancreatica (2/18 patients [11.1%]) when compared to the community group (5/587 patients [0.85%]) [7]. In a different study that looked at 3040 subjects who had ERCPs, 15 had ansa pancreatica. Of those 15, one had pancreatitis with a rate of 7% of those with ansa pancreatica having an episode of pancreatitis [7, 20, 21, 22].
In our case, there seemed to be no other explanation for the patient’s two episodes of AP other than the patient having ansa pancreatica. The patient had complete resolution of symptoms after sphincterotomy and stent placement [23]. Ansa pancreatica is thought to be the main cause of this patient’s AP or at least the main predisposing factor.
The relationship between ansa pancreatica and AP has not been well defined in the past. While the prevalence of ansa pancreatica in the literature is around 1%, there is very little research regarding its clinical relevance (Figure 6). There are several studies showing that ansa pancreatica increases one’s risk of AP [5, 7, 8, 10, 16] and findings from our case report are in line with previous studies. While ansa pancreatica is a rare anatomic variant, it is clear that it can predispose subjects to AP, especially those who already have underlying risk factors. Clinicians should be aware of this important anatomic variant and further investigate its presence especially in subjects with idiopathic recurrent AP.
The authors certify that they have no conflicts of interest, affiliation or involvement in any organization or entity in the subject discussed in this manuscript.