Research Article - (2023) Volume 7, Issue 1
Anlotinib Maintenance Following Adjuvant Chemotherapy in Newly Diagnosed Stage III-IV Patients with Epithelial Ovarian Cancer: A Retrospective Study
Jianghua Ding*
Department of Hematology and Oncology, The Affiliated Hospital of Jiujiang University, China
*Correspondence:
Jianghua Ding,
Department of Hematology and Oncology, The Affiliated Hospital of Jiujiang University,
China,
Email:
Received: 01-Feb-2023, Manuscript No. IPIPR-23-15608 ;
Editor assigned: 03-Feb-2023, Pre QC No. IPIPR-23-15608 (PQ);
Reviewed: 17-Feb-2023, QC No. IPIPR-23-15608;
Revised: 22-Feb-2023, Manuscript No. IPIPR-23-15608 (R);
Published:
01-Mar-2023, DOI: 10.21767/IPIPR.23.7.001
Abstract
Background and Aims: Anti-angiogenesis therapy with bevacizumab maintenance marginally improved the median
progression-free survival (mPFS) of 4.9 months in patients with advanced epithelial ovarian cancer (EOC). Anlotinib,
an oral small-molecular anti-angiogenic agent, has been reported to treat platinum-resistant EOC. However,
little is known about anlotinib maintenance therapy in newly diagnosed EOC.
Methods: This retrospective study included 20 patients with newly diagnosed EOC from a single hospital between
January 2020 and December 2021. The primary endpoints were mPFS, the overall response rate (ORR), and the
Disease Control Rate (DCR). Adverse reactions to therapy were also assessed.
Results: Among all EOC patients, the ORR was 65% (13/20) and the DCR was 95% (19/20), while the mPFS was
14.8 months (95% confidence interval, 11.5–18.0 months). Subgroup analysis revealed a trend toward a prolonged
PFS among EOC patients with a wild-type status compared to those harboring BRCA1/2 mutations (14.8 vs. 11.8
months, P=0.3621). Seven patients (35%) required a dose reduction because of grade 3 or 4 adverse events, which
were manageable and tolerable. No anlotinib-related death events were observed.
Conclusion: First-line anlotinib maintenance following adjuvant chemotherapy might be a novel therapeutic strategy,
especially for BRCA wild-type EOC patients.
Keywords
Anlotinib; Maintenance therapy; Newly diagnosed; Ovarian cancer
Introduction
According to cancer statistics, ovarian cancer has become a female
reproductive malignancy that severely threatens women’s health.
Ovarian cancer is the second leading cause of cancer-related death
in women among gynecologic malignancies in China. An estimated
57,090 new cases and 39,306 deaths are expected in China in 2022,
which is more than twice the number of cases expected in the United
States [1]. Due to the insidious onset of ovarian cancer, >70%
of patients is diagnosed at their first visit in an advanced stage of
the disease. The present standard clinical treatment for advanced
ovarian cancer includes primary debulking surgery, followed by
platinum-based adjuvant chemotherapy. However, >75% of patients
will relapse within 2 years of diagnosis and progressively develop
treatment resistances [2]. Of note, the relapse-free interval
is the key indicator for ovarian cancer survival following completion
of the recommended cycles of chemotherapy; this is also known as
the “platinum-free interval,” i.e., the length of time from the last platinum-based cycle to the time of disease progression [3]. Therefore,
extending the platinum-free interval may change the passive
act of waiting for recurrence into active prophylactic treatment,
which has become a topic of interest in the research on ovarian
cancer in recent years.
The National Comprehensive Cancer Network (NCCN) guideline
recommends first-line maintenance therapy using poly-ADP-ribose
polymerase (PARP) inhibitors, including olaparib, or niraparib
monotherapy for those with stage III–IV ovarian cancer following
completion of the recommended cycles of chemotherapy [4].
However, until 2021, PARP inhibitors like olaparib were covered by
the Chinese national health insurance system only for those with
BRCA1/2 mutations. Thus, they have not been widely used in China
due to the high cost of PARP inhibitors before 2021. In 2020, anti-
angiogenesis therapy with bevacizumab was recommended as a
maintenance treatment in ovarian cancer patients by the Chinese
Anti-cancer Association and NCCN guidelines, but bevacizumab
maintenance therapy only extends the Progression-Free Survival
(PFS) of 4.9 months without an Overall Survival (OS) benefit [5,6].
Furthermore, anlotinib, an oral small-molecular anti-angiogenic
agent, was reported to treat platinum-resistant ovarian cancer [7- 9]. Therefore, we retrospectively evaluated the efficacy and safety
of anlotinib maintenance therapy in patients with newly diagnosed
stage III–IV Epithelial Ovarian Cancer (EOC) after completing the
recommended cycles of adjuvant chemotherapy.
Patients and Methods
Patient Characteristics
From January 2020 to December 2021, a total of 20 patients with
newly diagnosed stage III–IV EOC were enrolled from the Affiliated
Hospital of Jiujiang University. The baseline characteristics of these
patients are described in Table 1. The median age at diagnosis was
60.5 years (range, 52-72 years). Pathological diagnosis confirmed
that 16 patients had serous carcinoma, 3 patients had endometrioid
carcinoma, and 1 patient had mixed serous and endometrioid
carcinoma. 12 patients were assessed for BRCA gene mutations (5
patients with mutant BRCA1/2 and 7 patients with wild-type BRCA),
while the other 8 patients did not undergo an examination of their
BRCA status. All patients were diagnosed with advanced-stage
disease (stage III, n=5; stage IV, n=15). 15 (75%) patients showed
malignant ascites and received intraperitoneal chemotherapy. 18
patients (90%) underwent cytoreductive surgery. 19 (95%) patients
were treated with 6 cycles of chemotherapy (paclitaxel and carboplatin),
except for 1 patient who received only 4 cycles of chemotherapy
owing to grade ≥ 3 myelosuppression.
| Characteristic |
n=20 (%) |
| Age, years |
| Median (range) |
60.5 (52–72) |
| ECOG PS, n (%) |
| 0 |
4 (20%) |
| 1 |
7 (35%) |
| 2 |
9 (45%) |
| Histology, n (%) |
| Serous |
16 (80%) |
| Endometrioid |
3 (15%) |
| Mixed serous and endometrioid |
1 (5%) |
| BRCA status, n (%) |
| BRCA 1/2 mutation |
5 (25%) |
| Wild type |
7 (35%) |
| Unknown |
8 (40%) |
| International FIGO stage |
| III |
5 (25%) |
| IV |
15 (75%) |
| Malignant ascites |
| Yes |
15 (75%) |
| No |
5 (25%) |
| Intraperitoneal chemotherapy |
| Yes |
15 (75%) |
| No |
5 (25%) |
| Cytoreductive surgery |
| Yes |
18 (90%) |
| No |
2 (10%) |
| Cycles of chemotherapy (paclitaxel and carboplatin) |
| 4 cycles |
1 (5%) |
| 6 cycles |
19 (95%) |
Table 1: Baseline characteristics of ovarian cancer patients (n (%)).
Treatment and Dose-Adjustment Protocols
Anlotinib (Nanjing Chia Tai Tian Qing Company, Nanjing, China)
was taken orally (before breakfast) once daily on days 1-14, every
3 weeks during a cycle. The doses of anlotinib were classified as 12
mg, 10 mg, and 8 mg daily. No chemotherapy or radiotherapy was
performed during anlotinib treatment. Anlotinib was taken until
disease progression or unacceptable toxicity.
According to the manufacturer’s instructions, drug dose-adjustments
are permitted based on the levels of adverse reactions, as
illustrated in Table 2. Adverse reactions were recorded and graded
from 0 to 4 according to the National Cancer Institute’s Common
Toxicity Criteria (NCI-CTC) version 4.0.
Levels of adverse reactions
(NCI-CTC) |
Dose adjustment |
| Grade 0–2 |
Drug administration will be continued at the initial dose of 12 mg/day as planned. |
| Grade 3 |
Drug administration will be paused, and anlotinib will be continued at the reduced dose of 10 mg/day when the NCI-CTC level is restored to <2 within 2 weeks. |
| Grade 4 |
Drug administration will be paused, and anlotinib will be continued at the reduced dose of 8 mg/day when the NCI-CTC level is restored to <2 within 2 weeks. If there is no recovery over 2 weeks, anlotinib will be permanently discontinued. |
Table 2: Principle for the adjustment of anlotinib dose.
Response Evaluation Criteria
The primary study outcome was PFS. The objective responses were
assessed according to the Response Evaluation Criteria in Solid Tumors
(RECIST) version 1.1, which were Complete Remission (CR),
Partial Remission (PR), Stable Disease (SD), and Progressive Disease
(PD). Gynecological examinations were conducted after every 2 cycles
of anlotinib treatment and included magnetic resonance and
abdominal color ultrasound imaging.
Follow-Up
All patients were followed-up with until disease progression or
death. The PFS was calculated from the start of anlotinib administration
until disease progression, patient follow-up loss, or death.
The end of the follow-up period was December, 31, 2022. The median
follow-up time of the entire group was 13.5 months (range,
6.0-21.0 months). No patients were lost to follow-up.
Statistical Analysis
Descriptive statistics were used to describe all participants’ clinical
characteristics. The Kaplan-Meier method was utilized to evaluate
PFS. All statistical analyses were performed using Graph Pad Prism
version 7.0 (Graph Pad Software, Inc., San Diego, CA, USA).
Ethics Approval and Consent to Participate
Ethics approval was obtained from the medical ethics committee
of Jiujiang University Affiliated Hospital. Informed consent was
waived because of the retrospective nature of the study. The study
was performed in accordance with the Declaration of Helsinki concerning
the ethical principles for medical research.
Results
Clinical Efficacy
Follow-up was completed for all 20 patients with advanced EOC.
The best response was evaluated using the RECIST (version 1.1) criteria
in all patients enrolled (Table 3). Only one patient achieved a
CR; 12 patients had a PR, and 6 patients reported SD; meanwhile,
1 patient experienced PD. The ORR and disease control rate (DCR)
were 65% and 95%, respectively. The median PFS (mPFS) was 14.8
months (95% confidence interval, 11.5-18.0 months) (Figure 1). In
addition, the wild-type subgroup showed a tendency toward a prolonged
mPFS compared to the BRCA1/2 mutation subgroup (14.8
vs. 11.8 months), but no significant difference was found between
them (P=0.3621) (Figure 2).
| Clinical outcome |
No. of patients (%) (n=20) |
| CR |
1 (5%) |
| PR |
12 (60%) |
| SD |
6 (30%) |
| PD |
1 (5%) |
| ORR (CR+PR) |
13 (65%) |
| DCR (CR+PR+SD) |
19 (95%) |
Table 3: Objective response in newly diagnosed EOC patients.
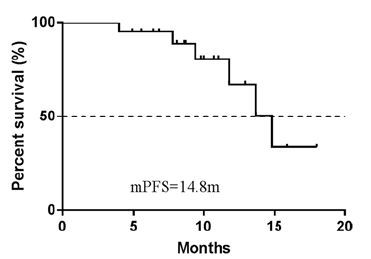
Figure 1: PFS curve of total population.
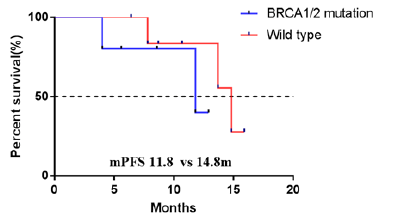
Figure 2: PFS curve of BRCA1/2 mutation and wild type subgroups.
Dose Adjustment
Seven patients (35%) were started on an initial dose of 10 mg/day,
but the doses of two patients were reduced to a daily dose of 8 mg
owing to grade 3 or 4 side effects. The other 13 patients (65%) began
treatment with 12 mg/day, but five required a dose reduction
to 10 mg/day due to grade 3 or 4 adverse events.
Adverse Effects
Adverse reactions were assessed from the start of anlotinib treatment
until disease progression or the last follow-up date. The anlotinib-
related adverse effects included hypertension, proteinuria,
fatigue, hand-foot syndrome, and leukopenia (Table 4). The grade 3
toxicities were hypertension (15%), fatigue (10%), leukopenia (5%),
and proteinuria (5%). Except for one case of grade 4 hypertension
and one case of grade 4 leukopenia, no grade 4 toxicities were recorded.
No deaths associated with anlotinib were observed.
| Adverse effects |
No. of patients |
Total |
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
| Hypertension |
7 (35%) |
4 (20%) |
3 (15%) |
1 (5%) |
15 (75%) |
| Proteinuria |
2 (10%) |
1 (5%) |
1 (5%) |
0 |
4 (20%) |
| Fatigue |
6 (30%) |
3 (15%) |
2 (10%) |
0 |
11 (55%) |
| Hand-foot syndrome |
5 (25%) |
2 (10%) |
0 |
0 |
7 (35%) |
| Leukopenia |
4 (20%) |
2 (10%) |
1 (5%) |
1 (5%) |
8 (40%) |
Table 4: Anlotinib-related adverse effects (n (%)).
Discussion
Although chemotherapy is initially effective for the treatment of
EOC, nearly all advanced-disease patients eventually relapse and
become resistant to platinum-based therapies within 5 years.
Therefore, how to extend the relapse-free interval is of great clinical
significance in improving the prognosis of ovarian cancer patients.
Currently, maintenance therapy has emerged as a novel
treatment strategy for advanced ovarian cancer patients, i.e., PARP
inhibitors (olaparib, rucaparib, and niraparib) and anti-angiogenesis
therapies (bevacizumab) [10].
In the PRIMA study, the mPFS of the niraparib group was markedly
longer than that of the placebo group (13.8 vs. 8.2 months) in
the overall population. Subgroup analysis revealed that the mPFS
was 22.1 and 21.9 months for the BRCA-mutation and Homologous-
Recombination Deficiency (HRD) groups, respectively. Of
note, the Homologous-Recombination Proficiency (HRP) subgroup
of ovarian cancer patients accounted for 65.7% of all patients with
an mPFS of 8.1 months [11]. In both GOG-0218 and ICON-7, firstline
bevacizumab maintenance only prolonged the mPFS by about
4.0 months for patients with advanced ovarian cancer, indicating a
limited efficacy of bevacizumab maintenance in extending the relapse-
free survival [12]. The phase III study of PAOLA-1 reported
that olaparib plus bevacizumab greatly improved the mPFS in comparison
to bevacizumab alone in ovarian cancer patients with HRD
(37.2 vs. 17.7 months), the prevalence of which was about 50%
[13]. However, about 50% of ovarian cancer cases had a BRCA-wild
type and HRP status, and these patients only marginally benefited
from PARP inhibitor maintenance therapy, with an mPFS of about
5 months [14,15]. Thus, there is an unmet need to extend the relapse-
free interval for patients with ovarian cancer, especially with
a BRCA-wild type and HRP status.
Anlotinib is an oral multi-targeted tyrosine kinase receptor inhibitor
that can inhibit tumor angiogenesis and growth in a wide range
of cancers. At present, the National Medical Production Administration
of China has approved anlotinib for posterior-line treatment
of advanced small-cell lung cancer, non-small-cell lung cancer,
esophageal carcinoma, and soft tissue sarcoma [16-19]. In 2015,
anlotinib was approved by the U.S. Food and Drug Administration
as an orphan drug for use in ovarian cancer treatment. Zhang et
al. reported that anlotinib monotherapy resulted in a marked PR
and a PFS of >4 months in an elderly patient with advanced EOC
after the failure of multiple-line chemotherapy [8]. In a retrospective
observational study, anlotinib alone, anlotinib combined with
chemotherapy, or the anti-programmed cell death protein 1 therapeutic
pembrolizumab was administered to 38 patients with platinum-
resistant or refractory EOC. The mPFS and median OS were
7.7 and 16.5 months, respectively. Among them, 17 patients receiving
anlotinib monotherapy achieved an mPFS of 7.7 months.
The ORR was 42.1%, while the DCR was 86.8% [20]. A prospective,
single-arm phase II study documented an ORR of 25% and a DCR
of 100% for a group of platinum-resistant EOC patients receiving
anlotinib plus pemetrexed [21]. The results indicated that anlotinib
exhibited moderate clinical outcomes for platinum-resistant EOC.
However, the subjects in these studies had chemo-resistant ovarian
carcinoma.
In this retrospective study, we first explored the effect of anlotinib
maintenance on the relapse-free interval of newly diagnosed EOC
patients. The ORR was 65%, and the DCR was 95%. Among all cases,
the mPFS time was 14.8 months, which is comparable to the
effect of niraparib (13.8 months) in the PRIMA study. Furthermore,
a subgroup analysis revealed that the mPFS was 11.8 months in the
BRCA1/2-mutation subgroup. Relatively speaking, anlotinib maintenance
was less effective against BRCA1/2-mutation EOC than
niraparib maintenance (22.1 months). However, anlotinib triggered
a satisfactory clinical efficacy for patients with BRCA wild-type EOC,
with an mPFS of 14.8 months, which was considerably better than
that of PRAP inhibitors (about 5 months). Additionally, 35% of all
patients experienced a dose reduction due to grade III-IV toxicities,
which was manageable and tolerable.
This study has some limitations that need to be addressed. First,
this was a retrospective study, which is a limitation. Second, the
overall sample size of this study was small, which needs to be expanded
further to validate the therapeutic findings. Finally, not all
patients had their BRCA-mutation status tested. However, we believe
that this study can provide a reference for anlotinib maintenance
therapy in newly diagnosed ovarian cancer patients.
Conclusion
In conclusion, first-line anlotinib maintenance therapy might be a
promising agent for ovarian cancer patients after they complete
the recommend cycles of chemotherapy, especially for BRCA wildtype
EOC patients with manageable toxicities. Further investigations
are warranted to confirm the clinical outcome of anlotinib
maintenance treatment following adjuvant chemotherapy in the
treatment of newly diagnosed EOC patients.
Funding
The authors have not disclosed any funding.
Author's Contributions
D.J. contributed to the study conception and design. Material
preparation, data collection and analysis were performed by D.J.
and L.Z. All authors read and approved the final manuscript.
Data Availability
The datasets used and/or analyzed during the current study available
from the corresponding author on reasonable request.
Acknowledgement
We thank LetPub (www.letpub.com) for its linguistic assistance
during the preparation of this manuscript.
Competing Interests Statement
The authors have not disclosed any competing interests.
References
- Xia C, Dong X, Li H, Cao M, Sun D (2022) Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin Med J (Engl). 135:584-590.
[Crossref] [Google Scholar] [Pubmed]
- Baert T, Ferrero A, Sehouli J, O'Donnell DM, González-Martín A, et al. (2021) The systemic treatment of recurrent ovarian cancer revisited. Ann Oncol. 32:710-725.
[Crossref] [Google Scholar] [Pubmed]
- Dockery LE, Rubenstein AR, Ding K, Mashburn SG, Burkett WC, et al. (2019) Extending the platinum-free interval: The Impact of omitting 2nd line platinum chemotherapy in intermediate platinum-sensitive ovarian cancer. Gynecol Oncol. 155:201-206.
[Crossref] [Google Scholar] [Pubmed]
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, et al. (2021) Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 19:191-226.
[Crossref] [Google Scholar] [Pubmed]
- Gonzalez MA, Oza AM, Embleton AC, Pfisterer J, Ledermann JA, et al. (2019) Exploratory outcome analyses according to stage and/or residual disease in the icon7 trial of carboplatin and paclitaxel with or without bevacizumab for newly diagnosed ovarian cancer. Gynecol Oncol. 152:53-60.
[Crossref] [Google Scholar] [Pubmed]
- Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, et al. (2015) Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 16:928-936.
[Crossref] [Google Scholar] [Pubmed]
- Ni J, Cheng X, Chen J, Guo W, Dai Z (2020) Anlotinib as exploratory therapy for platinum-resistant ovarian cancer: A retrospective study on efficacy and safety. Onco Targets Ther. 13:9857-9863.
[Crossref] [Google Scholar] [Pubmed]
- Sun L, Yang M, Zhang X, Li H, Wu L, et al. (2020) Anlotinib combined with etoposide for platinum-resistant recurrent ovarian cancer: A case report. Medicine (Baltimore). 99:e20053.
[Crossref] [Google Scholar] [Pubmed]
- Zhang P, Ma L, Wang X, Zhang R, Dong Y (2020) Successful treatment of advanced ovarian cancer with anlotinib: A case report. J Int Med Res. 48:1-7.
[Crossref] [Google Scholar] [Pubmed]
- Gogineni V, Morand S, Staats H, Royfman R, Devanaboyina M, et al. (2021) Current ovarian cancer maintenance strategies and promising new developments. J Cancer. 12:38-53.
[Crossref] [Google Scholar] [Pubmed]
- González-Martín A, Pothuri B, Vergote I, Christensen RD, Graybill W, et al. (2019) Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 381:2391-2402.
[Crossref] [Google Scholar] [Pubmed]
- Haunschild CE, Tewari KS (2020) Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer. Future Oncol. 16:225-246.
[Crossref] [Google Scholar] [Pubmed]
- Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, et al. (2019) Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 381(25):2416-2428.
[Crossref] [Google Scholar] [Pubmed]
- Matulonis UA, Walder L, Nøttrup TJ, Bessette P, Mahner S, et al. (2019) Niraparib maintenance treatment improves time without symptoms or toxicity (TWIST) versus routine surveillance in recurrent ovarian cancer: A twist analysis of the engot-ov16/nova trial. J Clin Oncol. 37:3183-3191.
[Crossref] [Google Scholar] [Pubmed]
- Tomao F, Vici P, Tomao S (2020) Expanding use of rucaparib as maintenance therapy in recurrent ovarian cancer: Updates from the ariel3 trial. Lancet Oncol. 21:616-617.
[Crossref] [Google Scholar] [Pubmed]
- Cai J, Zhou S, Luo Y, Liu A (2021) Effect and safety of anlotinib combined with s-1 for recurrent or metastatic esophageal cancer patients who refused or were intolerant to intravenous chemotherapy. Medicine (Baltimore). 100:e28126.
[Crossref] [Google Scholar] [Pubmed]
- Liu Y, Cheng Y, Wang Q, Li K, Shi J, et al. (2021) Effectiveness of anlotinib in patients with small-cell lung cancer and pleural effusion: Subgroup analysis from a randomized, multicenter, phase II study. Thorac Cancer. 12:3039-3045.
[Crossref] [Google Scholar] [Pubmed]
- Zhang RS, Liu J, Deng YT, Wu X, Jiang Y (2022) The real-world clinical outcomes and treatment patterns of patients with unresectable locally advanced or metastatic soft tissue sarcoma treated with anlotinib in the post-alter0203 trial era. Cancer Med. 11(11):2271-2283Crossref]
[Google Scholar] [Pubmed]
- Zhong Q, Liu Z (2021) Efficacy and safety of anlotinib in patients with advanced non-small cell lung cancer: A real-world study. Cancer Manag Res. 13:4115-4128.
[Crossref] [Google Scholar] [Pubmed]
- Cui Q, Hu Y, Ma D, Liu H (2021) A retrospective observational study of anlotinib in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Drug Des Devel Ther. 15:339-347.
[Crossref] [Google Scholar] [Pubmed]
- Chen J, Wei W, Zheng L, Ding J, Feng Y (2020) Phase II study of anlotinib plus pemetrexed for platinum-resistant epithelial ovarian cancer. Ann Oncol. 31:S630-S631.
[Crossref] [Google Scholar]
Citation: Ding J, Leng Z (2023) Anlotinib Maintenance Following Adjuvant Chemotherapy in Newly Diagnosed Stage III–IV Patients
with Epithelial Ovarian Cancer: A Retrospective Study. J Pharm Pharm Res. 7:001.
Copyright: © 2023 Ding J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source
are credited.



