Introduction
The ovine animal model is preferably used for cardiovascular interventions, e.g. implantations of medical devices and heart valve replacements. The cardiovascular parameters in sheep are highly comparable to those in humans with a similar cardiac index (sheep: 3.8 l/min/m2; humans: 3.0 l/min/m2), a similar blood volume (sheep: 60ml/kg; humans: 70ml/kg) and comparable hemodynamic parameters (heart rate: 100 beats per minute vs. 70 bpm, and a mean arterial blood pressure (MAP) of 92-98 mmHg vs. 120/80 (95) mmHg in humans [1,2].
To date, the sheep is irreplaceable as animal model in preclinical investigations in the field of cardiology and cardiac surgery. Every invasive cardiovascular procedure, e.g the implantation of heart valve prostheses, requires an efficient and suitable anticoagulation strategy and a comprehensive monitoring to ensure an unimpaired follow-up after intervention without thromboembolic events.
Details of specific requirements and features regarding anticoagulation strategies and specific knowledge about hemostasis in sheep are rare. Looked at more closely, even in human patients, the relationship between the AFXalevel and the efficacy of LMWH in prevention of VTE (venous thromboembolism) is still not clear. Particularly, the results of AFXa are highly variable and in combination with widely variable therapeutic ranges it can be stated that the prophylactic and therapeutic regime using LMWH is poorly standardized [3]. Most of the recent studies investigated heparin-induced suppression of intimal hyperplasia [4,5]. Others examined the administration of unfractionated heparin (UFH) with regard to the activated partial thromboplastin time (aPTT) and the activated clotting time (ACT) both not regarded as valid monitoring parameters for subcutaneously administered therapy [6]. In case of intravenous application, ACT and aPTT can be used to monitor dalteparin or enoxaparin [7].
A comparison of the anticoagulative properties of sheep with other mammalians reveals some remarkable differences in the ovine organism. First of all, hemorheologic properties differ by representing a decreased whole blood viscosity but a higher plasma fibrinogen level [6]. The van-Willebrand-Factor has a 3.5 times higher activity in comparison with humans [8].
Sheep show a rapid initiation of the contact activation pathway and high levels of factor VIII, but low levels of Protein C. Stronger clot firmness and a decreased capacity of clot lysis is documented in sheep as well [9].
Regarding the platelet inactivation, it is important to know that sheep need 10 times more prostacyclin than other species [10].
Notably sheep need more heparin than other species while the direct effect of heparin is higher.
Dalteparin as anticoagulative agent was chosen in accordance with one of our research partners (“LifeValve” project at UZH, Zurich, Switzerland), who also work with ovine models. They administered dalteparin after implantation of pulmonary valves but used a predefined dose according to manufacturer’s data of 5,000 IU once per day [11], which corresponds to a preventive dose in patients with an intermediate risk of VTE (venous thromboembolism) in patients with heart failure, NYHA II and NYHA III [5].
Dalteparin sodium is a sulphated polysaccharide obtained by a partial nitrous acid depolymerization from standard UFH of porcine origin with a mean molecular weight of 5.7 kD [12]. The advantages of dalteparin in comparison with UFH are a fast absorption and a high bioavailability after subcutaneous injection which is necessary only once daily and a high antithrombotic activity accompanied with lower incidence of heparin induced thrombocytopenia [12,13].
In many cases, the preventive administration of dalteparin recommends standardized doses for prevention and therapy of venous thromboembolism related to different risk stages [14,15]. In the current study, the target for AFXa was chosen according to the guidelines for prevention of VTE in patients with a moderate and high risk profile.
Low molecular weight heparins (LMWH) predominantly affect the AFXa, so it is appropriate to monitor their effect with an AFXa-assay, particularly in younger and pediatric patients, obese patients, during pregnancy and in patients with restriction of the renal function due to a potential accumulation (reduced elimination) and increased bleeding complications [13]. The documented anti-Xa activity in plasma is considered to be directly proportional to the plasma concentration of the administered LMWH. To determine the target dose of dalteparin, a blood collection for the determination of AFXa is necessary 4 hours after injection when the peak of plasma concentration is reached (Figure 1).
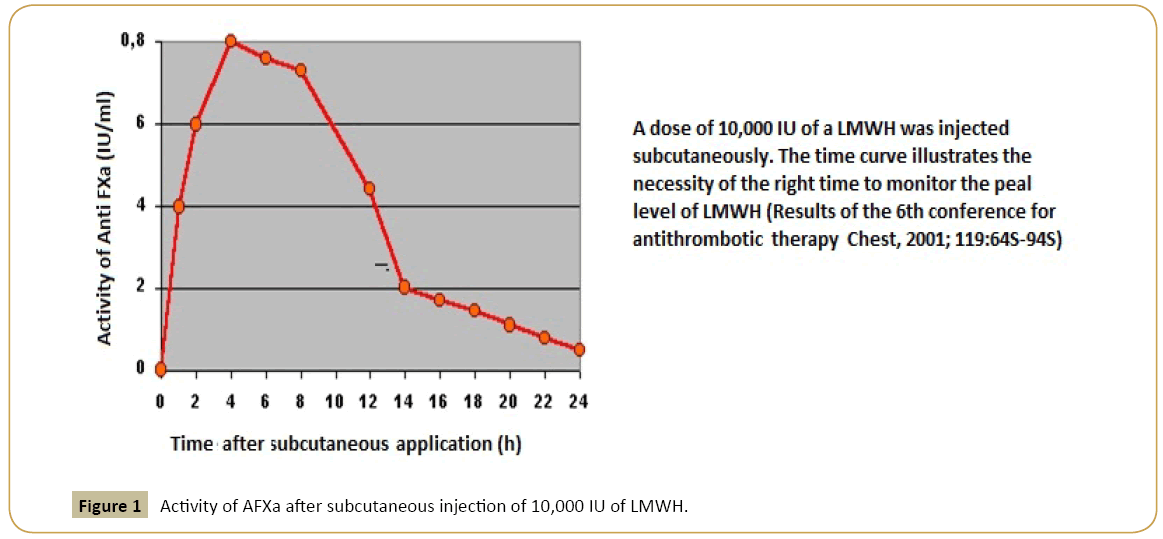
Figure 1: Activity of AFXa after subcutaneous injection of 10,000 IU of LMWH.
Our original reasearch work describes (A) a dose-finding study of dalteparin in three sheep for the aim of preventing thromboembolic events in interventions with a high general risk of emboli. This dose was (B) applied and evaluated in four sheep undergoing transvenous valve implantation.
Methods
The animal experiments were approved by the regional authorities (Landesamt für Gesundheit und Soziales, Berlin, Germany). Approval for the animal experiment was given by the ethics committee of the responsible veterinary department for animal welfare at the regional office for Health and social affairs under the registration number G0111/11 from September, 2015. German regulations for care and treatment of laboratory animals were adhered permanently (FELASA, GV-SOLAS).
Three (dose-finding study) plus four (valve implantation) fullygrown healthy grey horned heath ewes (mean body weight 43.4 kg) were housed in an appropriate stable at the veterinary care unit of the department for experimental medicine of the Charité, University Hospital of the Humboldt-University of Berlin. The sheep came from two different sheep breeders in the greater Berlin area where they were held under comparable conditions. At our unit, daily examinations, standardized vaccinations and deworming was conducted to ensure their healthy state. Animals were held in a group-housed pen with free access to ad libitum water and food.
At the day of cardiac intervention, the sheep were kept from feed and water for twelve hours before being treated with an intramuscular premedication containing 0.4 mg/kg midazolame (Dormicum® Roche, Germany), butorphanol 0.4 mg/kg (Alvegesic®, Alvetra, Germany) and 0.03 mg/kg atropine (Atropinsulfat® 0.5 mg/ml, B. Braun, Germany) and sedation with ketamin (0.5 mg/kg). The animals were positioned on their right side. The left perijugular region was prepared with an antiseptic and local anesthesia with 2.0 ml lidocaine 2% (Lidocain 4%, B. Braun, Melsungen) A pediatric double-lumen central intravenous catheter (4 Fr, length of introduction 8 cm; Arrow® Int. Corporate) was placed in the left jugular vein via Seldinger technique and fixated with a skin suture (Vicryl® 2-0, Ethicon, Johnson & Johnson, Norderstedt, Germany). The puncture site was covered with a sterile wound dressing, bandaged additionally with a water-andsoil- resistant elastic cohesive dressing (Vetrap®, 3M-Medica, Germany) and padded with cotton wool (Rolta-Soft®, Hartmann, Germany) around the sheep’s neck.
Shortly after intervention, sheep were able to stand up and feed. The sample for baseline values of anti-FXa, AT III and aPTT, as well the retention levels for urea and creatinine at the beginning of the investigation, were collected with a blood collecting system (Baxter Vacutainer®, Unterschleißheim, Germany) for citrate blood and serum. Before filling the particular tubes, 5.0 ml of aspirated blood was discarded. After securing the sample, the lumen was purged with 5.0 ml sterile saline 0.9% and locked with 0.3 ml trisodium citrate (Citra-Lock 30%®, Dirinco B. V., The Netherlands) afterwards and the lumen was relocked with a sterile closure plug (Kombi LL®, Fresenius Kabi, Germany). All blood samples were collected according to this procedure.
After placing the catheter and collecting blood for the baseline level, 250 IU dalteparin were injected subcutaneously in the lateral cervical region in week one at 7.00 AM on five consecutive days. In the second week, 350 IU dalteparin were injected subcutaneously at 7.00 AM on 5 consecutive days.
4 hours after application, the maximum level (peak) of anti-FXa in blood was determined, 12 hours and 24 hours after the injection of dalteparin, the levels of anti- FXa in blood were monitored again. The determination of AFXa and AT III as baseline level on day 1 of the first and second examination week, respectively, were monitored at the laboratory of the DHZB, the levels for aPTT every day, collected four hours after dalteparin administration, were analyzed at Synlab laboratories in Berlin (Synlab Vet®, Labor Berlin, Germany) and every other determination of AFXa (4, 12 and 24 hours after administration) were performed at the laboratory of DHZB as well. Every day the puncture site of the central venous cathether was inspected visually, disinfected (Softasept N®, Braunovidon® Salbe, B.Braun, Melsungen, Germany) and bandaged with a new wound dressing.
The venous catheter was removed on day 5 of each week after the blood collection, 12 hours after injection of dalteparin. The catheter was removed and the puncture site was compressed manually. Local anesthesia with 2.0 ml lidocaine 4% was administered and the puncture site was closed using an absorbable superficial skin suture (Vicryl® 2-0, Ethicon, Johnson & Johnson, Norderstedt, Germany).
After a two-day recovery period the animals underwent the same procedures in the second examination week with a dose of 350 IU dalteparin per day.
After finishing the dose finding study, 4 grey horned heath sheep received pulmonary valve prostheses in a minimally invasive procedure. They were treated with the weight adapted, optimized dose of dalteparin for a period of 5 days after intervention. Those animals underwent a follow up period of 52 weeks with monthly intracardiac echocardiography for functionality and morphological findings of the implanted graft. After the completed follow-up period of one year, animals were euthanized and the heart was explanted for post-mortem investigation of the graft.
Results
The minimum requirement for achieving an anti-F-Xa-level >0.1 IU/ml at 12 hours after dalteparin injection in high-risk patient [13] was realized both in the first and second investigation week. The mean anti-F-Xa-range 12 hours post-injection in week 1 was 0.13 IU/ml plasma, the mean anti-F-Xa-level 12 hour post injection in week 2 was 0.20 IU/ml plasma (Figures 2 and 3).
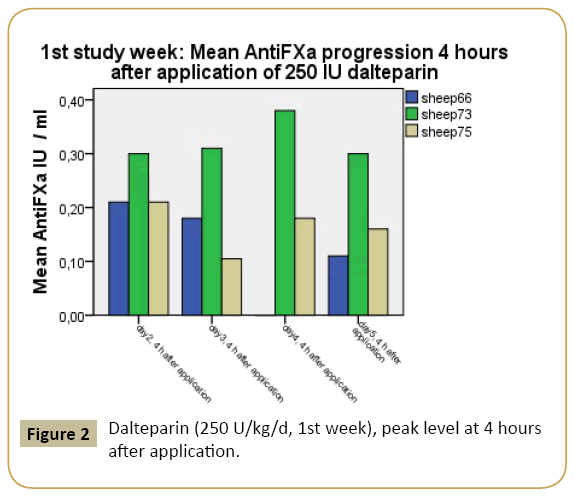
Figure 2: Dalteparin (250 U/kg/d, 1st week), peak level at 4 hours after application.
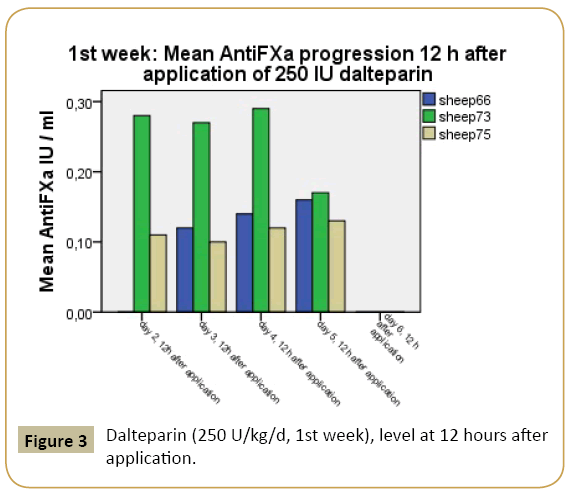
Figure 3: Dalteparin (250 U/kg/d, 1st week), level at 12 hours after application.
The mean peak concentrations of dalteparin-related AFXa in plasma were 0.36 IU/ml in week 1 and 0.66 IU/ml in week 2. The peak level in week 1 is associated with the target for prevention of venous thromboembolism with low risk. The mean level for the peak target in week 2 is sufficient for prevention of venous thromboembolism concerning moderate to high-risk patients (Figures 4 and 5).
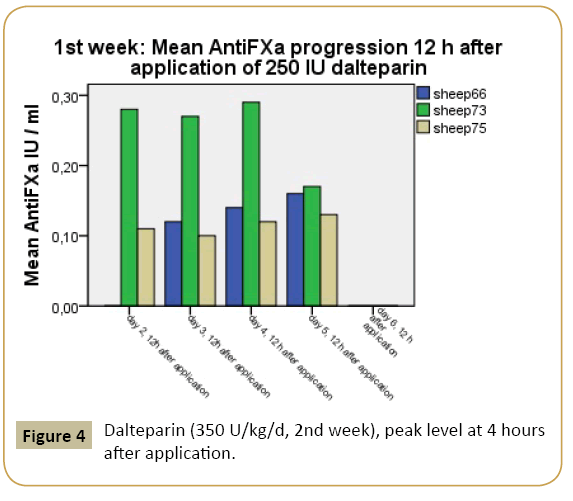
Figure 4: Dalteparin (350 U/kg/d, 2nd week), peak level at 4 hours after application.
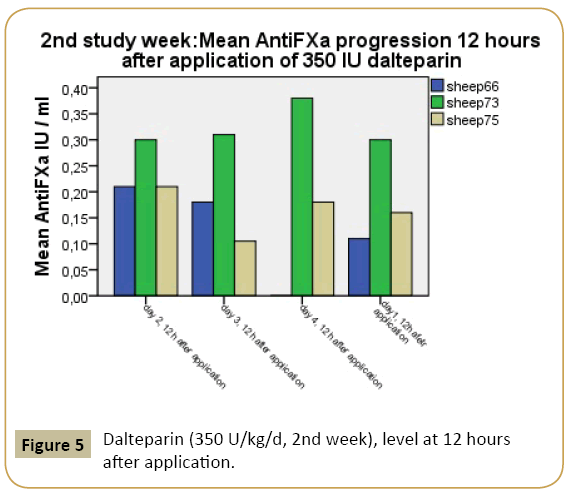
Figure 5: Dalteparin (350 U/kg/d, 2nd week), level at 12 hours after application.
Parameters of renal function, thrombocytes and values for PTT and ATIII at begin of the study were in normal range (Table 1).
| |
N |
minimum |
maximum |
mean |
Standard deviation |
variance |
| Urea(baseline,1stweek) |
3 |
4.05 |
5.29 |
4.6200 |
0.62 |
0.392 |
| Creatinine(baseline,1stweek) |
3 |
62.80 |
72.20 |
67.63 |
4.70 |
22.143 |
| Thrombocytes(baseline,1stweek) |
3 |
248,000 |
459,000 |
349,333 |
105,7475 |
1,11810 |
| PTT_baseline, 1stweek |
3 |
27.20 |
34.00 |
29.87 |
3.62 |
13.17 |
| ATIII(baseline, 1stweek) |
3 |
61.00 |
73.00 |
67.67 |
6.11 |
37.33 |
| PTT_4h_post (eachday) |
3 |
36.20 |
43.60 |
38.90 |
4.08 |
16.69 |
Table 1: Results for renal parameters, thrombocytes and PTT and AT III.
Creatinine showed a positive correlation towards the AFXa in the second week of investigation.
The body weight showed a positive correlation towards the peak of AFXa not in the first, but in the second investigation week and a positive correlation with the baseline creatinine and baseline AT III both in the first and second investigation week. Demonstrating a linear regression model, peak levels from the first and the second week showed a significant linear relation towards the body weight of sheep (Figures 6 and 7).
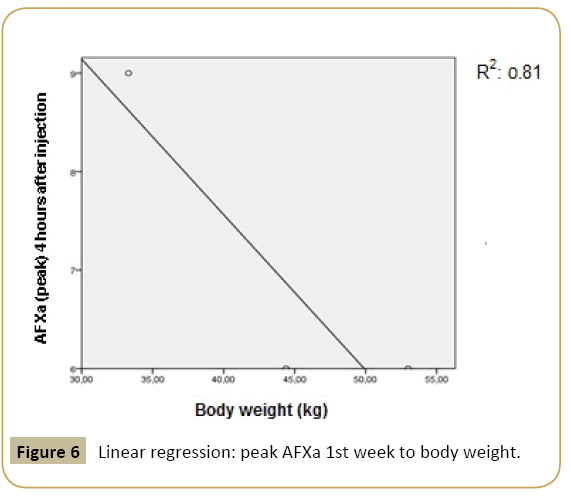
Figure 6: Linear regression: peak AFXa 1st week to body weight.
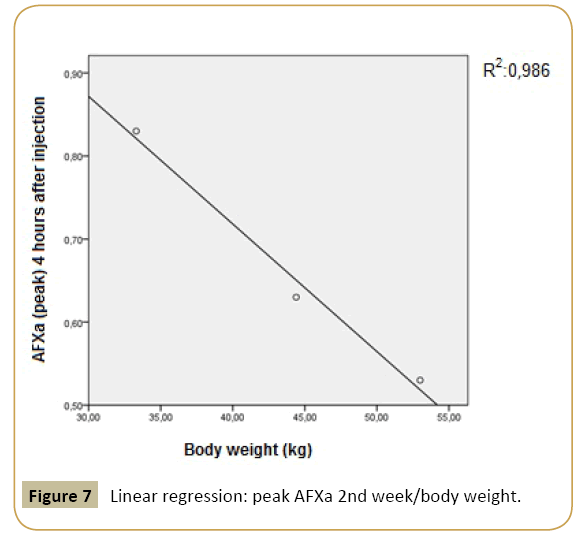
Figure 7: Linear regression: peak AFXa 2nd week/body weight.
Taking in account the above results, for the second study part (valve implantation) the dalteparin dose of 350 U/kg/d was chosen. In all four animals, evaluation showed no signs of thrombosis or embolic events over 52 weeks follow-up.
Discussion
Planning the implantation of heart valves in sheep, an optimal post interventional anticoagulation strategy is required. In the lack of having specific knowledge concerning the specific requirements of sheep obtaining LMWH, we conducted the previous study.
When comparing sheep with other animals or humans, it became obvious that sheep require higher doses of LMWH for an effective anticoagulation.
Comparability referred to the hemostasis in different animal species
Today, the sheep is an accepted and widely used animal model to evaluate and characterize cardiovascular prostheses. However, only a few available information regarding normal ranges of AFXa for prevention of VTE using dalteparin led to the current study with the aim of presenting a reliable anticoagulation regimen in sheep using dalteparin.
Platelets and the coagulation system are essential for hemostasis, and their interaction is the determining factor in blood compatibility with heart valve prostheses.
While evaluating such devices through various animal models, it is important to recognize species variation for proper extrapolation of the results of animal experiments to humans [16]. The same authors evaluated and compared platelet and coagulation function in humans and three different animal species (calf, sheep, and dog), using a newly developed clot signature analyzer (CSA) which support the simultaneously measurement of platelet and coagulation function. The results suggested that the canine species showed distinctively shorter time values than other species, including humans, indicating that the dog was not an ideal animal model for the evaluation of blood–surface interactions. Another important difference was seen in platelet counts which were significantly higher in calves and sheep than in dogs and humans. Several studies have favored the sheep model as an acceptable animal model for testing the blood compatibility of cardiovascular implants [11]. Connell et al. [17] recently compared hemostasis in sheep versus goats. In this study, bolus heparin was given followed by continuous infusion. In general, a higher heparin dose was necessary in goats than in sheep. The results indicated that sheep and goats can be safely and effectively anticoagulated with heparin. A previous study performed in the early 1970s [10], the coagulation values between mature sheep and normal human adults were compared. The significant differences affecting the hemostatic system were the markedly decreased fibrinolytic activity and the increased platelet number and adhesiveness in sheep. These factors could increase the tendency toward hypercoagulability. In a different study, Goodman [18] compared the number of platelets and morphology of surface-adhered platelets on various foreign surfaces under static in vitro conditions, reporting that ovine platelets were much less reactive than human platelets. The fact that ovine platelets were much less active must be taken into account in the evaluation of cardiovascular prostheses tested in sheep.
Body weight and dosage of dalteparin
In a previous study including humans, it could be demonstrated that with once-daily weight based dosing, the peak effect of dalteparin on day 3 of investigation was similar in three weight categories, between normal weight and obese patients [19]. Nevertheless, in the current investigation, we assessed a weight dependence of the administered dosage of dalteparin, represented with a linear relation between peak level of dalteparin and the body weight. Nevetheless it is ascertained that body weight in sheep is very variable in sheep because of the varying amounts of fluids and ingesta in the segmented stomachs. Therefore, weight related doses are accompanied with unreliability in sheep.
Recommended dosage of dalteparin
The target peak and trough anti-Xa level therapeutic ranges for LMWH therapy for the treatment of venous thromboembolism are controversial. Furthermore, there have been no studies that have prospectively validated such ranges especially for sheep. For dalteparin, the manufacturer recommends the peak anti-Xa level be 0.5-1.0 for intermediate to high-risk patients. However, this range was developed based on twice-daily dosing.
Renal function and pharmacodynamics of dalteparin
Changes in dalteparin pharmacodynamics were well documented in critically ill patients with renal insufficiency [12]. In the previous study, animals did not have any limitations of renal function, but it could be demonstrated that the level of AFXa showed a positive correlation towards the baseline creatinine for the purpose of an a Figure 5 Dalteparin (350 U/kg/d, 2nd week), level at 12 hours ltered pharmacologic response.
Conclusions
To administer the appropriate dosage of dalteparin in animals, a laboratory monitoring is desirable. In comparison with the results in humans in a prospective study with adult trauma patients [13], the incidence of developing DVT and VTE was significantly lower when AFXa was adjusted using a defined protocol.
The quantity of required dalteparin to achieve the prophylactic range of AFXa-units in plasma for patients with a high risk for developing venous thromboembolism is disproportionately higher in sheep, in comparison with humans.
Anti-factor-Xa levels should be monitored in sheep during a preventive administration of LMWH in consideration of the instable efficacy in the ovine organism. Weight- related doses should be adapted for the particularly requested target level. In case of difficulties for achieving the required target, AT III levels should be monitored. The parameter aPTT is not a suitable parameter for controlling the efficacy of preventive administration of dalteparin but might be suitable as parameter for detecting LMWH overdosage.
Steady–state of sufficient treatment using dalteparin can be attained on the second day of administration.
Study limitations
First of all, the target ranges for anti-factor-Xa levels in plasma were borrowed from implications for humans. The transferability for sheep is conceivably limited.
The present study was implemented in healthy animals, without any limitations in mobility or severe illness, no further activation of coagulation and no preceded implantation of any devices. Therefore, the comparability to interventionally treated animals, however named (devices, valve replacement), was not fulfilled. Otherwise, evaluating the efficacy of LMWH treatments requires the testing in healthy sheep before an actually demand. It is inevitable for a basic comprehension of the dalteparinconcerned mode of action in sheep in advance.
A main limitation of the study is the small-sized study group including only three female animals, which restricts the validity of the investigation. Further investigations with a greater study population of different age, a greater range of different weights and gender should be included in further studies.
Another difficulty consists in the fact that the current investigation was conducted with grey horned heaths, and the transferability to other breeds is potentially limited. Further investigation is necessary to enlarge the existing skills concerning LMWH anticoagulation in sheep.
Acknowledgments
This work was financially supported by the European Union`s Seventh Framework Program (FP 7/2007-2013) under Grant Agreement Number 242008 (LifeValve). We thank Katharina Weber, Stefan Schröder, Kerstin Fiebig and Juliane Wilhelm for their valuable help in the animal experiments. For proof reading we owe thanks to our copy-editor Anne Gale.
Funding Source
EU-Grant 242008, acronym: LifeValve
Author contributions
KB, HS and BS designed the study. KB, HS, and BS carried out the experiments. KB carried out the data sampling. KB, HS, and BS evaluated data. KB and HS performed the statistical analysis. KB and BS wrote the manuscript. All authors read and approved the final manuscript.
References
- Lin HC (2012) Monitoring and treatment for perioperative complications. Western Veterinary Conference, Auburn University, Auburn, Alabama, USA
- Scoggins BA, Coghlan JP, Denton DA (1984) ACTH-induced hypertension in sheep. In: Handbook of Hypertension, Vol 4 Experimental and Genetic Models of Hypertension, Edited by W de Jong, Elsevier Science Publishers BV Amsterdam, 107-134.
- Simoneau MD, Vachon A, Picard F (2010) Effect of prophylactic dalteparin on anti-factor Xa levels in morbidly obese patients after bariatric surgery. ObesSurg 20: 487-491.
- Fletcher JP, Ao PY, Hawthorne WJ (2004) Antiproliferative effects of low molecular weight heparin. ANZ J Surg 74: 793-796.
- Taylor A, Fletcher JP, Ao PY (1996) Inhibition of fibro-intimal hyperplasia in a polytetrafluoroethylene vascular graft with standard heparin and low molecular weight heparin. Aust N Z J Surg 66: 764-767.
- Funk DM (2012) Coagulation assays and anticoagulant monitoring. Hematology Am SocHematolEduc Program 2012: 460-465.
- Cavusoglu E, Lakhani M, Marmur JD (2005) The activated clotting time (ACT) can be used to monitor enoxaparin and dalteparin after intravenous administration. J Invasive Cardiol 17: 416-421.
- Park SG, Kim SC, Choi MJ, Lee HS, Min BG, et al. (2003) Heparin monitoring in sheep by activated partial thromboplastin time. Artif Organs 27: 576-580.
- Foley SR, Solano C, Simonova G, Spanevello MM, Bird RJ, et al. (2014) A comprehensive study of ovine haemostasis to assess suitability to model human coagulation. Thromb Res 134: 468-473.
- Gajewski J, Povar ML (1971) Blood coagulation values of sheep. Am J Vet Res 32: 405-409.
- https://www.pfizer.de/medikamente-produkte/pfizer- produkte/detailansicht/fragminR-7500-ie10000-ie-12500-ie15000-ie18000-ie- fertigspritze.htm
- Hull RD, Pineo GF, Francis C, Bergquist D, Fellenius C, et al. (2000) Low-molecular weight heparin prophylaxis using dalteparin in close proximity to surgery vs. warfarin in hip arthroplasty patients: a double-blind, randomized comparison. The North American Fragmin Trial Investigators. Archive of Internal medicine 160: 2199-2207.
- Droege ME, Mueller EW, Besl KM, Lemmink JA, Kramer EA, et al. (2014) Effect of a dalteparin prophylaxis protocol using anti-factor Xa concentrations on venous thromboembolism in high-risk trauma patients. J Trauma Acute Care Surg 76: 450-456.
- Kani C, Markantonis SL, Nicolaou C, Maggina N (2006) Monitoring of subcutaneous dalteparin in patients with renal insufficiency under intensive care: an observational study.J Crit Care 21: 79-84.
- Sato M, Harasaki H: Evaluation of platelet and coagulation function in different animal species using the xylum clot signature analyzer. 2002, American Society for Artificial Internal Organs 360-364.
- Read MS, Potter JY, Brinkhous KM (1983) Venom coagglutinin for detection of von Willebrand factor activity in animal plasmas. J Lab Clin Med 101: 74-82.
- Connell JM, Khalapyan T, Al-Mondhiry HA, Wilson RP, Rosenberg G, et al. (2007) Anticoagulation of Juvenile Sheep and Goats With Heparin, Warfarin and Clopidogrel. Pediatric Mechanical Circulatory Support, American Society for Artificial Internal Organs 229-237.
- Goodman SL (1999) Sheep, pig, and human platelet-material interactions with model cardiovascular biomaterials. J Biomed Mater Res 45: 240-250.
- Wilson SJ, Wilbur K, Burton E, Anderson DR (2001) Effect of Patient Weight on the Anticoagulant Response to Adjusted Therapeutic Dosage of Low-Molecular- Weight Heparin for the Treatment of Venous Thromboembolism. Hemostasis 31: 42-48